The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Wanling Xie presented the results of ICECaP-2, which aimed to validate metastasis-free survival (MFS) as a surrogate for overall survival (OS) in a contemporary cohort of patients with localized prostate cancer.
Previous work from the Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP) group, published by Xie et al. in The Journal of Clinical Oncology,1 has previously validated MFS as a strong surrogate for OS in patients with localized prostate cancer, using data from 19 trials (n=12,712). 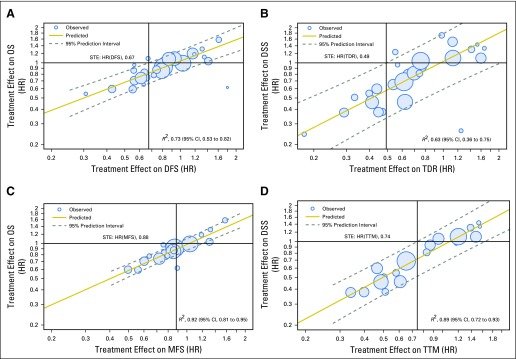
However, this analysis was performed using data from trials of patients treated predominantly before 2004, when docetaxel became available for mCRPC.2 As such, the authors sought to ‘re-validate’ the surrogacy of MFS for OS in a more contemporary cohort of patients during an era of increased availability of docetaxel and androgen receptor pathway inhibitors (ARPIs) for metastatic castrate-resistant prostate cancer (mCRPC).
Eligible trials for ICECaP-2 included those meeting the following eligibility criteria:
- Providing individual patient data following the publication of ICECaP-2
- Evaluating adjuvant/salvage therapy for localized prostate cancer
- Collected MFS and OS data
MFS was defined as distant metastases or death from any cause, and OS as death from any cause. Surrogacy was evaluated using a meta-analytic two-stage validation model, with an R2 ≥ 0.7 defined a priori as clinically relevant, using an approach similar to that used in the preceding ICECaP publication.1
ICECaP-2 included 14 trials, with individual-level patient data from 15,164 patients.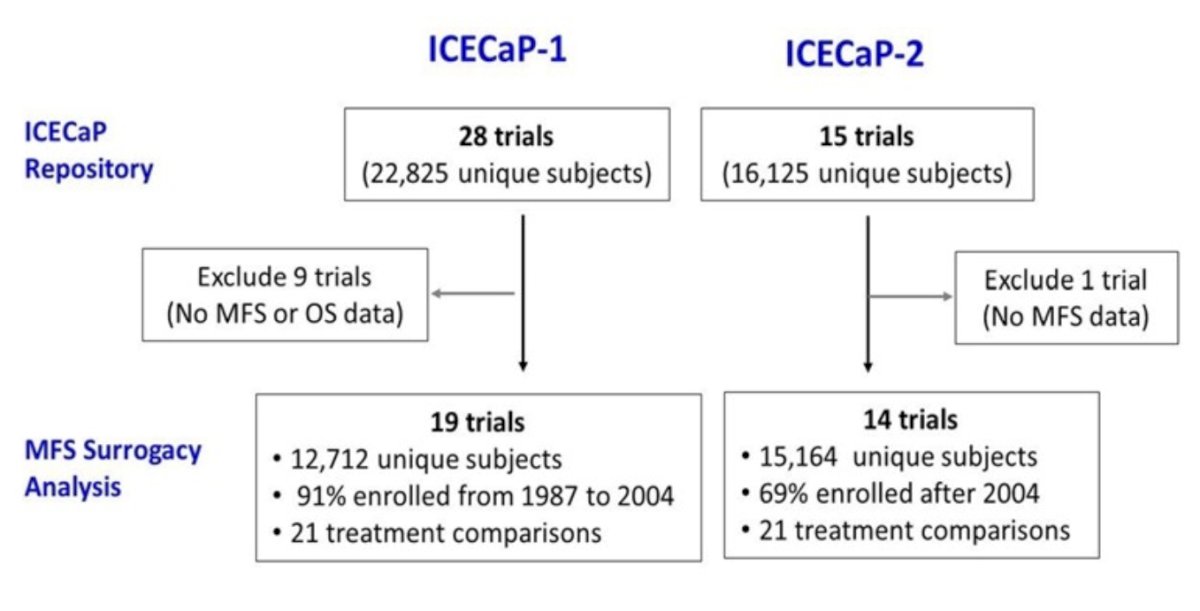
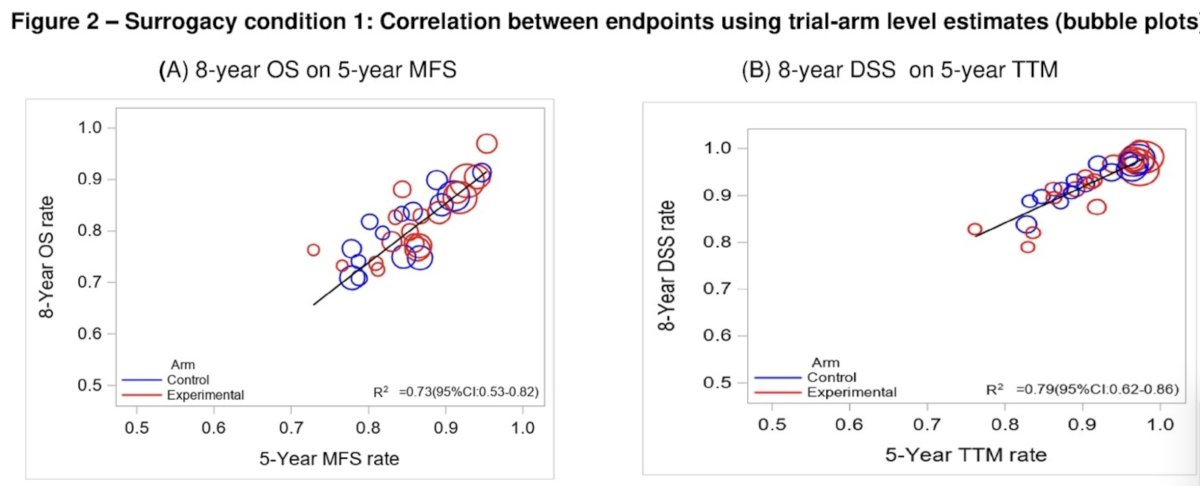
For surrogacy condition 1, Kendall’s tau was 0.92 for MFS with OS at the patient-level, and R2 from weighted linear regression (WLR) of 8-yr OS on 5-yr MFS was 0.73 (95% CI: 0.53 to 0.82) at the trial level.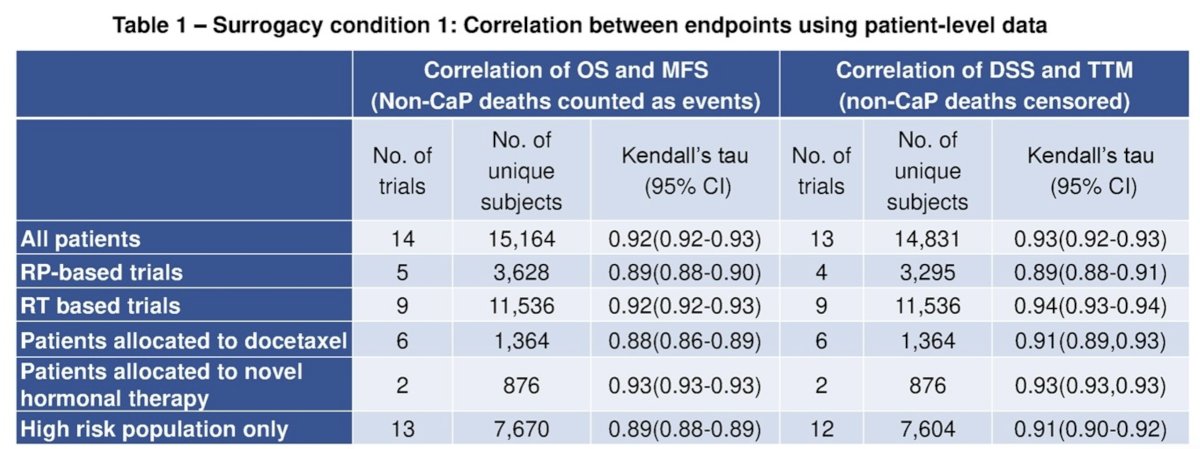
For condition 2, R2 was 0.83 (95% CI: 0.64 to 0.89) from WLR of log (HR)-OS on log (HR)-MFS. The surrogate threshold effect on OS was an HR(MFS) of 0.81. 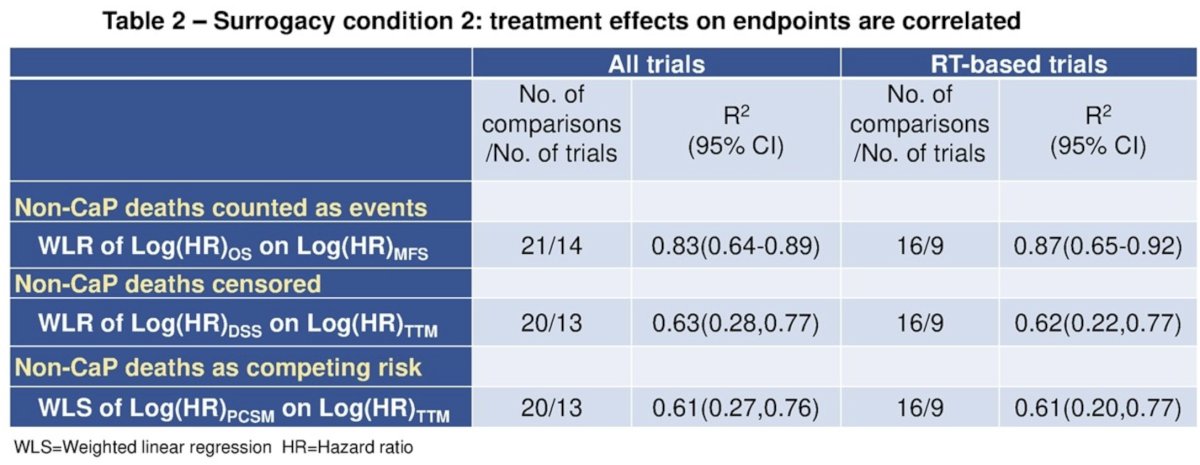

The table below compares trial characteristics and surrogacy outcomes between ICECaP-1 and ICECaP-2. We note that ICECaP-2 included more contemporaneous trial cohorts (2010-2016: 29% versus 1%), and the median time from metastasis to death was meaningfully longer (3.1 versus 1.9 years in favor of ICECaP-2), which is likely secondary to the recent increased availability of life-prolonging therapies in the advanced prostate cancer space. 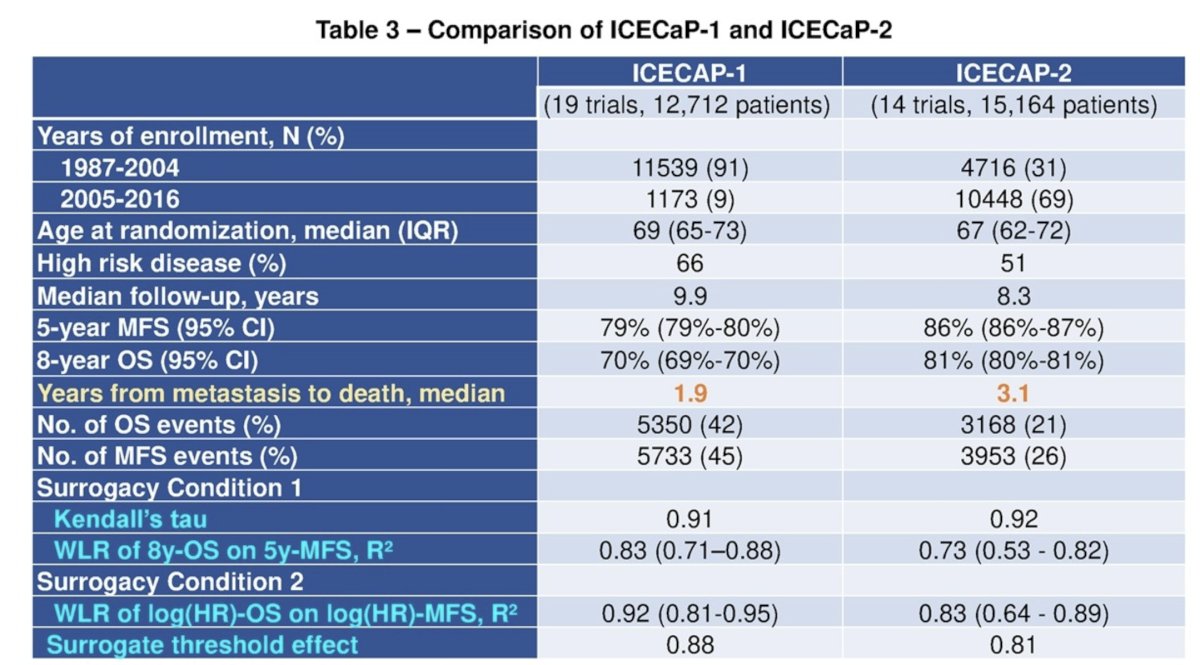
The authors concluded that MFS remains a valid surrogate for OS localized prostate cancer patients treated in a contemporary era with increased availability/access to docetaxel, ARPIs, and other life-prolonging therapies for mCRPC. The surrogacy effects were similar to those observed in ICECaP-1. These results support the continued use of MFS as the primary outcome measure for ongoing trials in localized prostate cancer.
Presented by: Wanling Xie, MS, Statistician, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Xie W, Regan MM, Buyse M, et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol. 2017;35(27):3097-3104.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512.


