(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Stephen Freedland presented results of the secondary analysis of EMBARK, specifically those pertaining to enzalutamide in combination with leuprolide acetate for the treatment of prostate cancer patients with high-risk biochemical recurrence.
EMARK (NCT02319837) is a phase 3 randomized study of enzalutamide or placebo plus leuprolide acetate and enzalutamide monotherapy in high-risk biochemically recurrent prostate cancer. The study design is summarized below. In brief, all patients had a PSA ≥1 ng/ml after RP or ≥2 ng/ml above nadir after primary EBRT, with a PSA doubling time (PSADT) of ≤9 months. Patients had no evidence of metastasis on conventional imaging and baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSADT, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide acetate (blinded arm) 22.5 mg every 12 weeks
- Placebo + leuprolide acetate (blinded)
- Enzalutamide monotherapy 160 mg (unblinded)
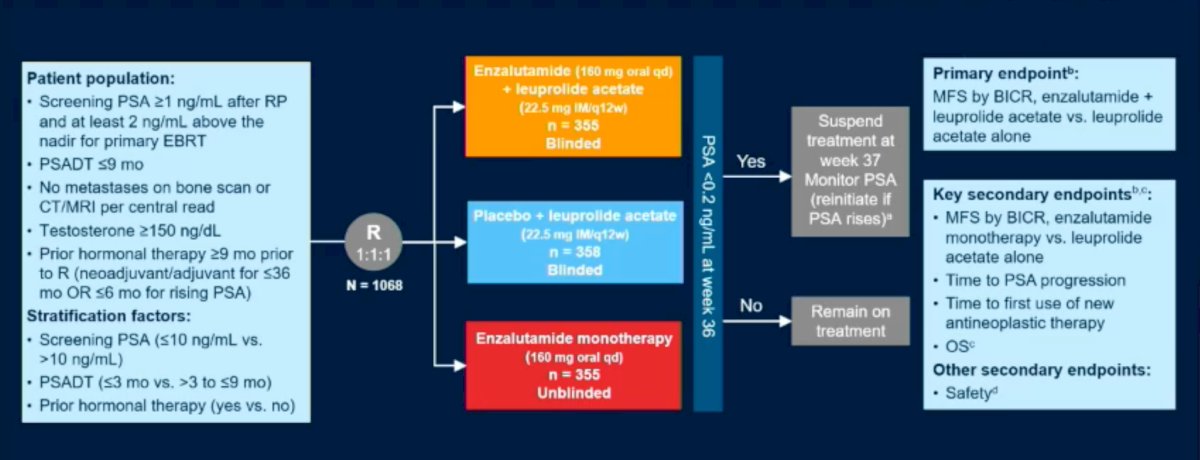
PSA was assessed at 36 weeks, and if patients had:
- PSA<0.2 Treatment was suspended at week 37 and PSA monitored with treatment reinitiated if PSA rose again (≥2 ng/mL for patients with primary radical prostatectomy, and ≥5 ng/mL for patients with radiotherapy)
- PSA>0.2 Treatment was continued
The initial results of EMBARK were presented at AUA 2023. At a median follow-up of 5 years, the combination of enzalutamide/leuprolide, versus leuprolide alone, demonstrated a significant improvement in MFS (HR: 0.42, 95% CI: 0.31 – 0.61, p<0.0001). The median MFS was not reached in either arm as of date.
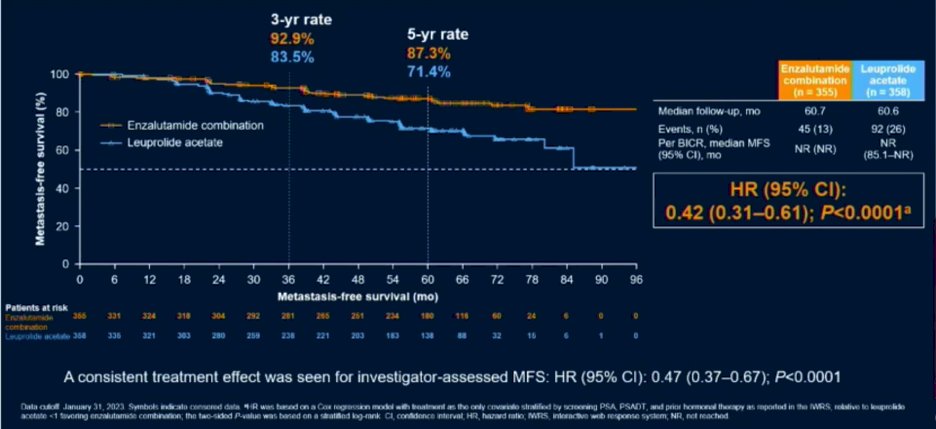
This combination arm also demonstrated an OS improvement (HR: 0.59, 95% CI: 0.38 – 0.90, p=0.0142). While the 95% CI upper bound did not cross the null value of 1 and the p-value was <0.05, the OS outcome was not yet mature and had not yet met the pre-specified efficacy boundary of p<0.0001.
In this updated analysis, Dr. Freedland presented additional secondary efficacy endpoints for combination enzalutamide + leuprolide acetate versus placebo + leuprolide acetate, which included time to:
- Distant metastasis
- Resumption of any hormonal therapy after treatment suspension
- Castration resistance
- Symptomatic progression
- First symptomatic skeletal event.
This secondary analysis included 713 patients, of whom 355 and 358 were randomized to enzalutamide + leuprolide acetate and placebo/leuprolide acetate, respectively. The median treatment duration, excluding treatment suspension, was 32.4 months for enzalutamide + leuprolide acetate and 35.4 months for placebo/leuprolide acetate. Compared to leuprolide acetate alone, combination enzalutamide +leuprolide acetate was associated with significant improvements in time to:
- Distant metastasis (HR=0.44; 95% CI: 0.28 to 0.69, p=0.0002)
- Resumption of any hormonal therapy after treatment suspension (HR=0.69, 95% CI: 0.58 to 0.83, p<0.0001)
- Castration resistance (HR=0.09, 95% CI: 0.05 to 0.16, p<0.0001)
- Symptomatic progression (HR=0.55; 95% CI: 0.43 to 0.70, p<0.0001)
- First symptomatic skeletal event (HR=0.26, 95% CI: 0.13 to 0.55, p=0.0001)
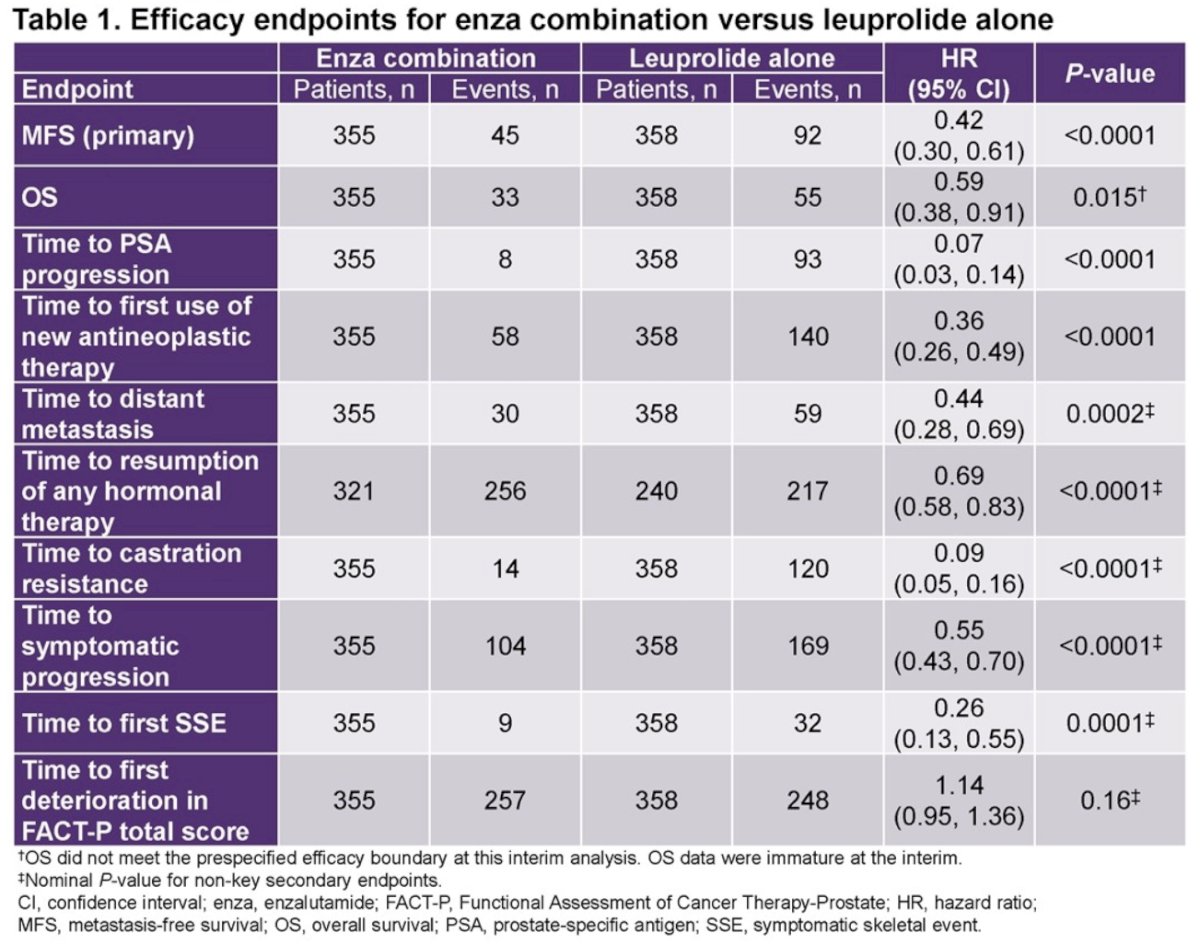
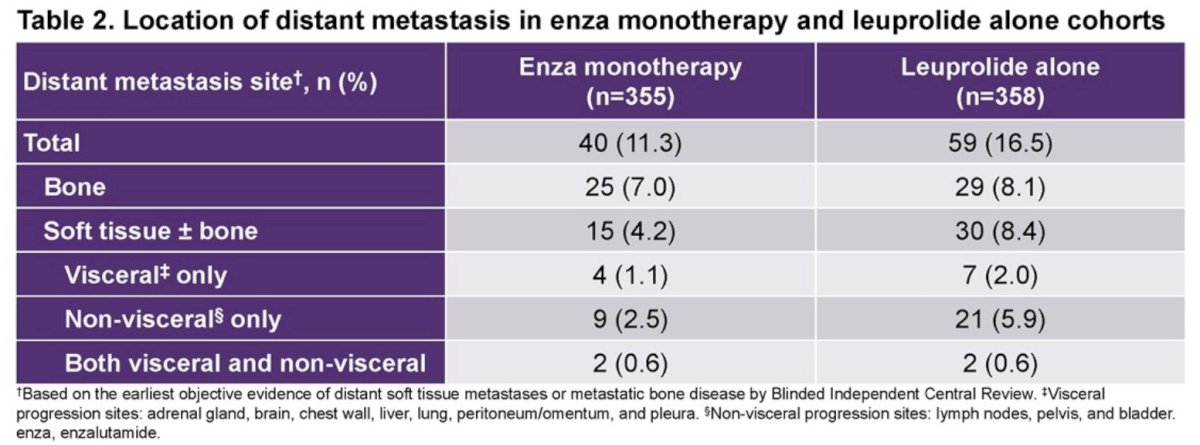
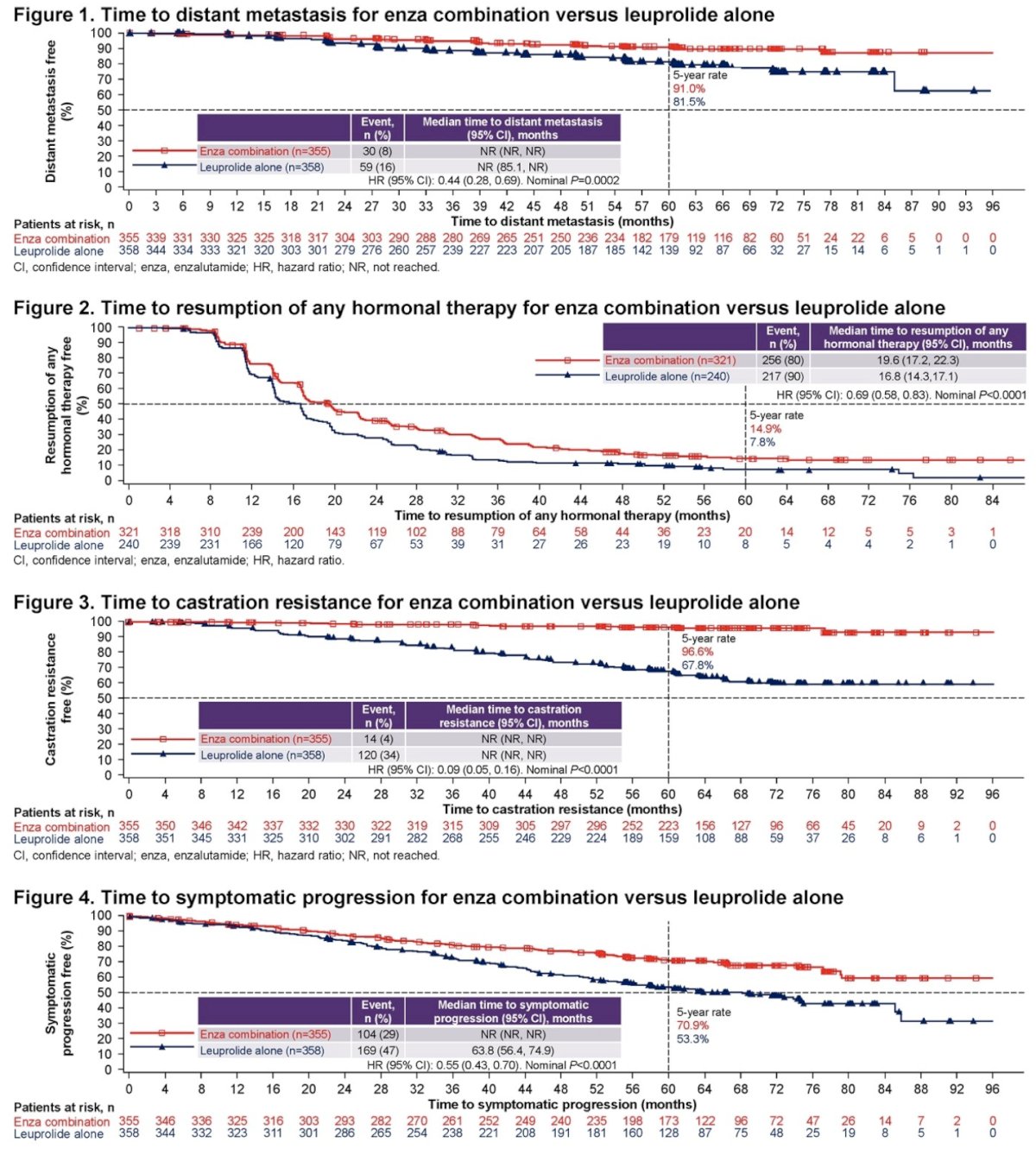
Based on these results, the authors concluded that in patients with high-risk biochemical recurrence, combination enzalutamide plus leuprolide acetate shows clinically meaningful delays in time to distant metastasis, resumption of any hormonal therapy after treatment suspension, castration resistance, symptomatic progression, and first symptomatic skeletal event. Dr. Freedland suggested that if approved, enzalutamide combination may represent a new standard of care for patients with high-risk biochemical recurrence.
Presented by: Stephen Freedland, MD, Professor, Department of Urology, Cedars Sinai Hospital, Los Angeles, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


