(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Jeroen Jansen presented the results of a distributional cost-effectiveness analysis evaluating the health inequality impact of darolutamide for non-metastatic castrate-resistant prostate cancer (nmCRPC).
Darolutamide was approved for the treatment of patients with nmCRPC and a short PSA doubling time based on the results of the ARAMIS trial that demonstrated that the addition of darolutamide to ADT, compared to placebo, was associated with significant improvements in metastasis-free survival (40.4 versus 18.4 months).1 Darolutamide was shown to be safe and effective for Non-Hispanic Black patients, who appear to be disproportionately affected by nmCRPC in the US. Given the improved public awareness of health equality issues in the medical care of advanced cancer patients, the impact of a new intervention on inequality in health outcomes is increasingly viewed as an element in health technology assessment. The objective of this study was to quantify the health inequality impact of darolutamide plus androgen-deprivation therapy (ADT) relative to ADT for nmCRPC patients in the U.S. using a distributional cost-effectiveness analysis.
Using a decision model, the quality-adjusted life years (QALYs) and costs were estimated for Non-Hispanic White, Non-Hispanic Black, Asian, and Hispanic patients. The magnitude of differences in QALYs among these subgroups was expressed using the Atkinson relative inequality indices (0 = equal outcomes and 1 = maximum inequality between subgroups) for both strategies. Their difference was defined as the inequality impact of darolutamide among treated patients. Subtracting equally distributed health opportunity costs from the QALY gains with darolutamide facilitated calculation of the overall health inequality impact across population subgroups.
Darolutamide plus ADT was associated with an additional 1.04 (95% CI: 0.57 to 1.49) QALYs per treated patient compared to ADT alone, with the greatest gain observed among Non-Hispanic, Black patients at 1.48 QALYs (95%CI: 0.49 to 2.72).


The relative inequality in QALYs among patients decreased by 66% from an inequality score of 0.032 (95% CI: 0.004 to 0.080) with ADT to 0.011 (95% CI: 0.000 to 0.049) with darolutamide plus ADT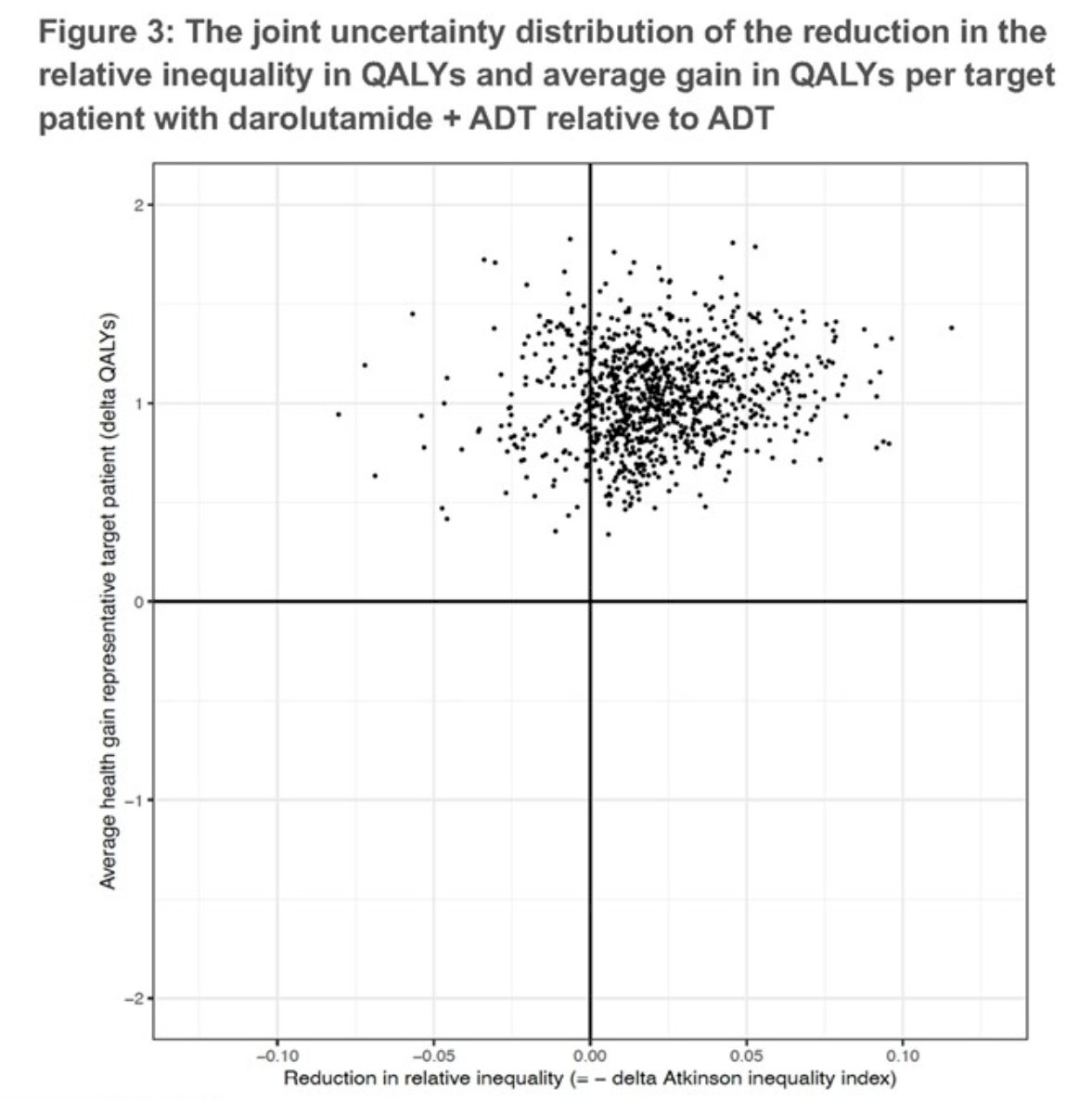
When health opportunity costs are factored in, the treatment of eligible nmCRPC patients with darolutamide resulted in the largest net gain in QALYs among the Non-Hispanic, Black patients population, thereby having a favorable impact on inequalities in quality adjusted life expectancy. 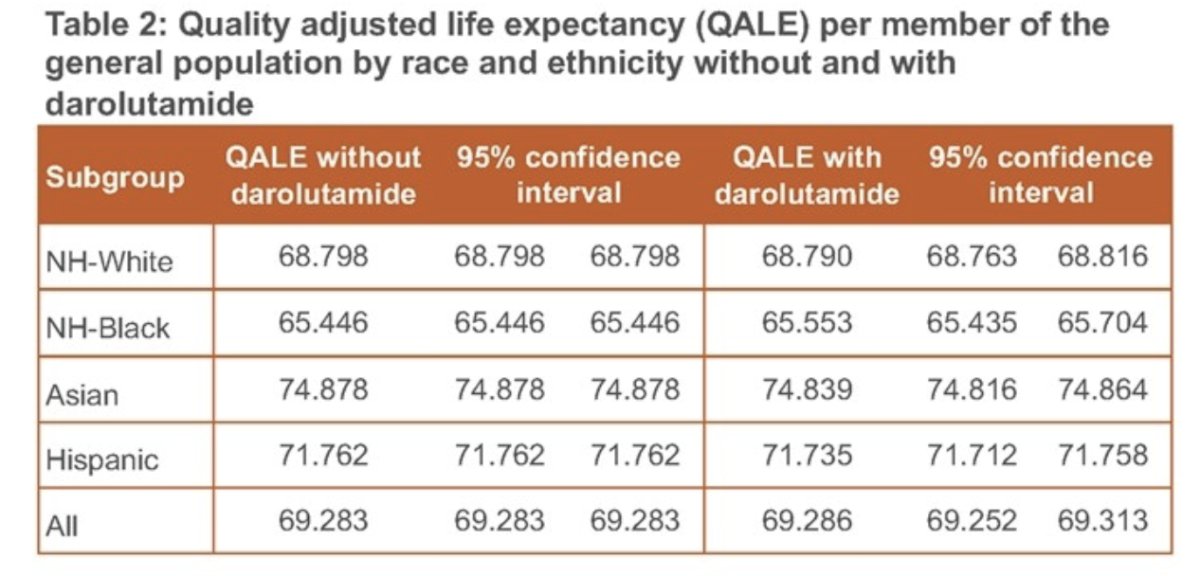
Dr. Jansen concluded that darolutamide plus ADT for the treatment of nmCRPC results in improved health outcomes compared to ADT alone and reduces inequality in health outcomes across racial and ethnic subgroups in the US.
Presented by: Jeroen Jansen, MD, PhD, Associate Professor, Clinical Pharmacy, School of Pharmacy, University of California, San Francisco, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.


