(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Jose Arranz Arija presented the efficacy results from PROSTRATEGY, a SOGUG pilot randomized trial of ADT plus docetaxel +/- nivolumab or ipilimumab/nivolumab in patients with high-volume metastatic hormone sensitive prostate cancer (mHSPC).
Although the GETUG-AFU 15 trial, initially published in February 2013,1 failed to demonstrate a survival benefit to the addition of docetaxel to ADT in men with mHSPC (HR 0.88, 95% CI 0.68 to 1.14), subsequent data from the CHAARTED trial (HR 0.61, 95% CI 0.47 to 0.80)2 and the first analysis of the multi-stage STAMPEDE trial (HR 0.80, 95% CI 0.65 to 0.99)3 both demonstrated significantly improved overall survival amongst men receiving docetaxel with a median survival difference of more than 12 months. The results of these seminal studies established doublet therapy with docetaxel + ADT as a standard of care option for mHSPC patients.
Immune checkpoint inhibitors have demonstrated significant benefits in many cancer types, but to date, no clinically meaningful benefits have been observed in the advanced prostate cancer space. The objective of PROSTRATEGY was to evaluate whether the addition of ipilimumab and/or nivolumab could improve clinical outcomes when added to docetaxel + ADT.
PROSTRATEGY was designed as a randomized (2:1:1) multi-arm, multi-stage trial aimed at comparing ADT + docetaxel (75 mg/m2 x6 cycles) versus:
- ADT + docetaxel followed by nivolumab (3 mg/Kg every 2 weeks x 12 months) OR
- ADT + ipilimumab + docetaxel followed by nivolumab (ADT + ipilimumab 1 mg/Kg every 3 weeks x 2 cycles followed by docetaxel x3 cycles → ipilimumab 3mg/Kg every 3 weeks x2 cycles → docetaxel x3 cycles → nivolumab x 12 months).
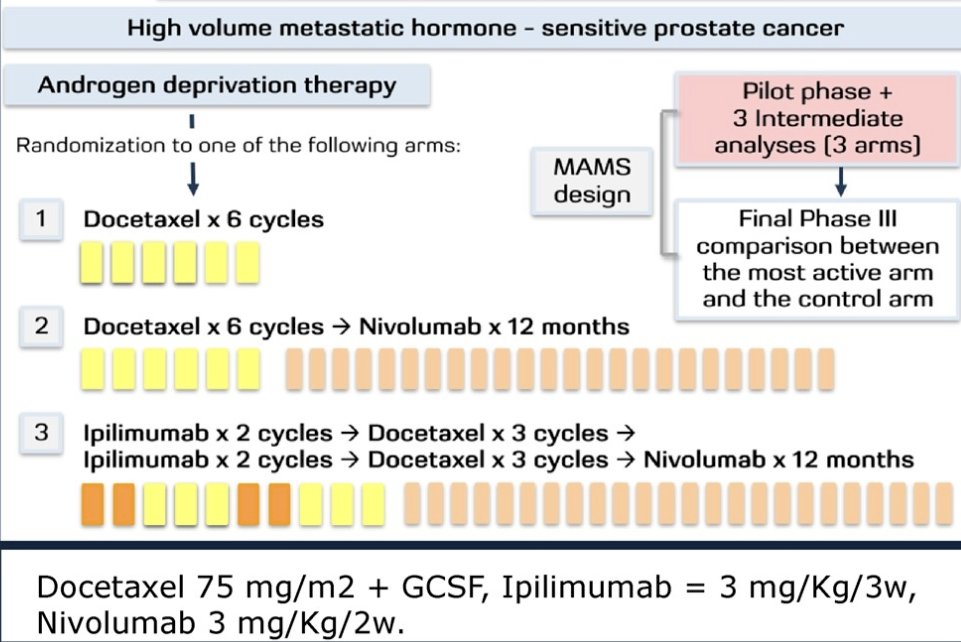
This trial included patients with high-volume mHSPC from 30 academic centers in Spain. The objectives of the pilot phase were to assess feasibility, safety and efficacy outcomes in terms of radiographic and PSA response, radiographic and clinical progression-free survival or death (rPFS, cPFS), time to castration-resistant prostate cancer or death (TCRPC), time to symptomatic skeletal-related event (TSSRE) and overall survival (OS) from the first dose of ADT, using both both RECIST1.1 + Prostate Cancer Working Group 3 (PCWG3) and the immune therapy-adapted iRECIST + PCWG3 criteria.
To date, 150 patients have been randomized to:
- Docetaxel arm (n=75)
- Docetaxel + nivolumab (n=37)
- Docetaxel + ipilimumab + nivolumab (n=38)
The baseline patient characteristics were as follows:
- Mean patient age was 66.4 years (39% older than 70)
- 98% were Caucasian
- 99.3% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 – 1
- 88% had de novo (i.e., synchronous) metastases
- Mean PSA was 157 ng/ml
- 88% had Gleason ≥ 8 disease
- 31% had visceral involvement
- 17% received local therapy to the prostate (no differences between arms)

At a median follow-up of 32.5 months from ADT initiation, 60% of the patients had progressed and 41.3% died. Overall, there were no significant differences in efficacy outcomes between patients in the three arms. A PSA50 response was most commonly seen in the docetaxel + nivolumab arm (97.3% versus 88% and 76.3% in the docetaxel and docetaxel + ipilimumab + nivolumab arms, respectively). A complete or partial response was more commonly observed in the docetaxel + nivolumab (43.2%) and docetaxel+ ipilimumab + nivolumab arms (44.7%) versus the docetaxel arm (34.7%), however only 12% of patients had evidence of progressive disease in the docetaxel arm, compared to 19 – 26% in the other two arms.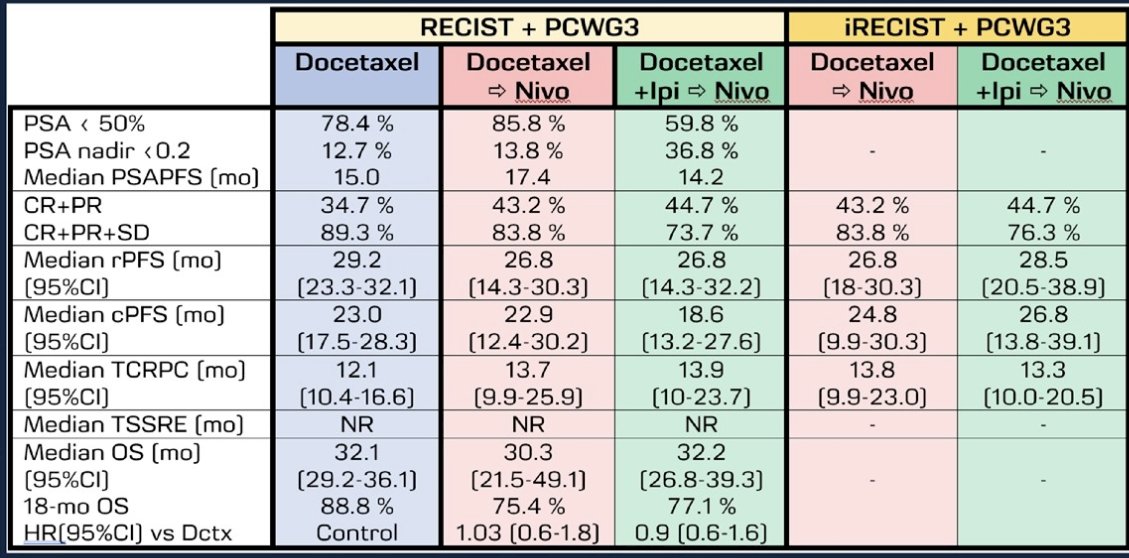
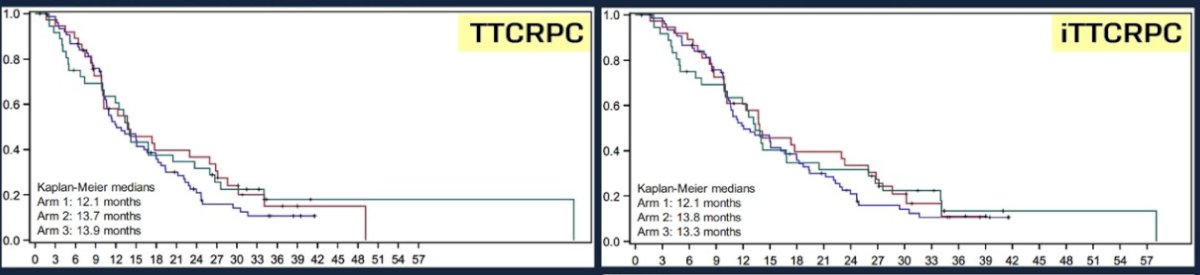
The median time to CRPC was longest in the docetaxel + nivolumab arm (13.7 months). Median OS was highest in the docetaxel arm (32.1 months versus 29.5 and 30.4 months).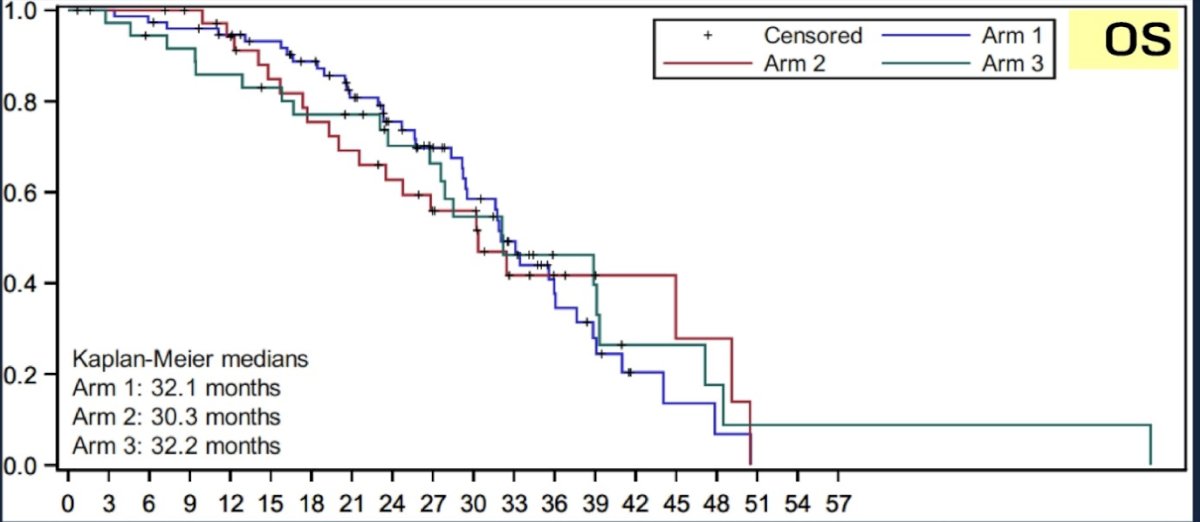
Dr. Arranz Arija and colleagues concluded that while the addition of ipilimumab +/- nivolumab to ADT + docetaxel is feasible, to data there are no statistically or clinically meaningful benefits to this treatment intensification and, thus, continuation of this study is not justified. The results seem to be similar irrespective of whether the PCWG3 plus either RECIST or iRECIST criteria are used, although some differences between both methods may appear with longer follow-up.
Presented by: Jose A. Arranz Arija, MD, Medical Oncology, General University Hospital Gregorio Marañón, Madrid, Spain,
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.


