(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Axel Merseburger presented the results of a secondary analysis of TITAN that evaluated the effect of rapid ultra-low PSA decline in patients with metastatic castration-sensitive prostate cancer (mCSPC) who received apalutamide + ADT.
TITAN was a double-blind, phase 3 trial, that randomized mCSPC patients to apalutamide (240 mg per day) or placebo, added to ADT. Prior docetaxel usage was permitted (1.2% of the study cohort). 81% of patients presented with de novo mCSPC and 62.7% of patients had CHAARTED high volume disease. The first reported outcomes of this trial demonstrated a significant benefit for ADT + apalutamide (HR: 0.67, 95% CI: 0.51 to 0.89),1 and with longer follow-up (median follow-up of 44.0 months) an overall survival (OS) benefit with the addition of apalutamide (HR: 0.65, 95% CI: 0.53 to 0.79) has been maintained, despite 39.5% of patients in the placebo arm crossing over to the treatment arm.2
In a recent ad hoc analysis of TITAN, Chowdhury et al. demonstrated that apalutamide-treated patients were significantly more likely to achieve PSA responses/declines (≥50% or ≥90% from baseline or to ≤0.2 ng/ml). Significantly, achievement of a deep PSA decline at landmark 3 months of apalutamide treatment was associated with longer OS (HR: 0.35, 95% CI: 0.25 - 0.48), rPFS (HR: 0.44, 95% CI: 0.30 - 0.65), time to PSA progression (HR: 0.31, 95% CI 0.22 - 0.44), and time to castration resistance (HR: 0.38, 95% CI: 0.27 - 0.52) compared with no decline (p < 0.0001 for all).3 As such, the objective of this study was to evaluate the association of ultra-low PSA decline with clinical outcomes.
This analysis included 525 and 527 patients receiving apalutamide and placebo, respectively. Two groups of ultra-low PSA were defined:
- UL1: PSA 0.02 – 0.2 ng/ml
- UL2: PSA ≤0.02 ng/ml
The associations between ultra-low PSA grouping and the following outcomes were evaluated using landmark analysis, Kaplan-Meier estimates, and Cox proportional hazards modeling:
- OS
- rPFS
- Time to castration resistance
- Time to PSA progression
UL2 PSA decline (i.e., ≤0.02 ng/ml) was achieved by 49% and 17% of patients in the apalutamide and placebo arms, respectively. UL1 and UL2 PSA response by treatment arm at 3 and 6 months were achieved by:
- 3 months:
- Apalutamide: 38% and 23%
- Placebo: 15% and 5%
- 6 months:
- Apalutamide: 29% and 36%
- Placebo: 17% and 6%

Of note, apalutamide patients with UL1/UL2 response at 3 months had lower baseline PSA and higher % of low-volume disease, compared to those with PSA>0.2 ng/ml at 3 months. Patients with UL1/UL2 at 3 or 6 months had significantly improved long-term outcomes irrespective of disease volume. Volume-adjusted outcomes were significantly improved in patients with UL1/UL2 values achieved at 3 or 6 months.
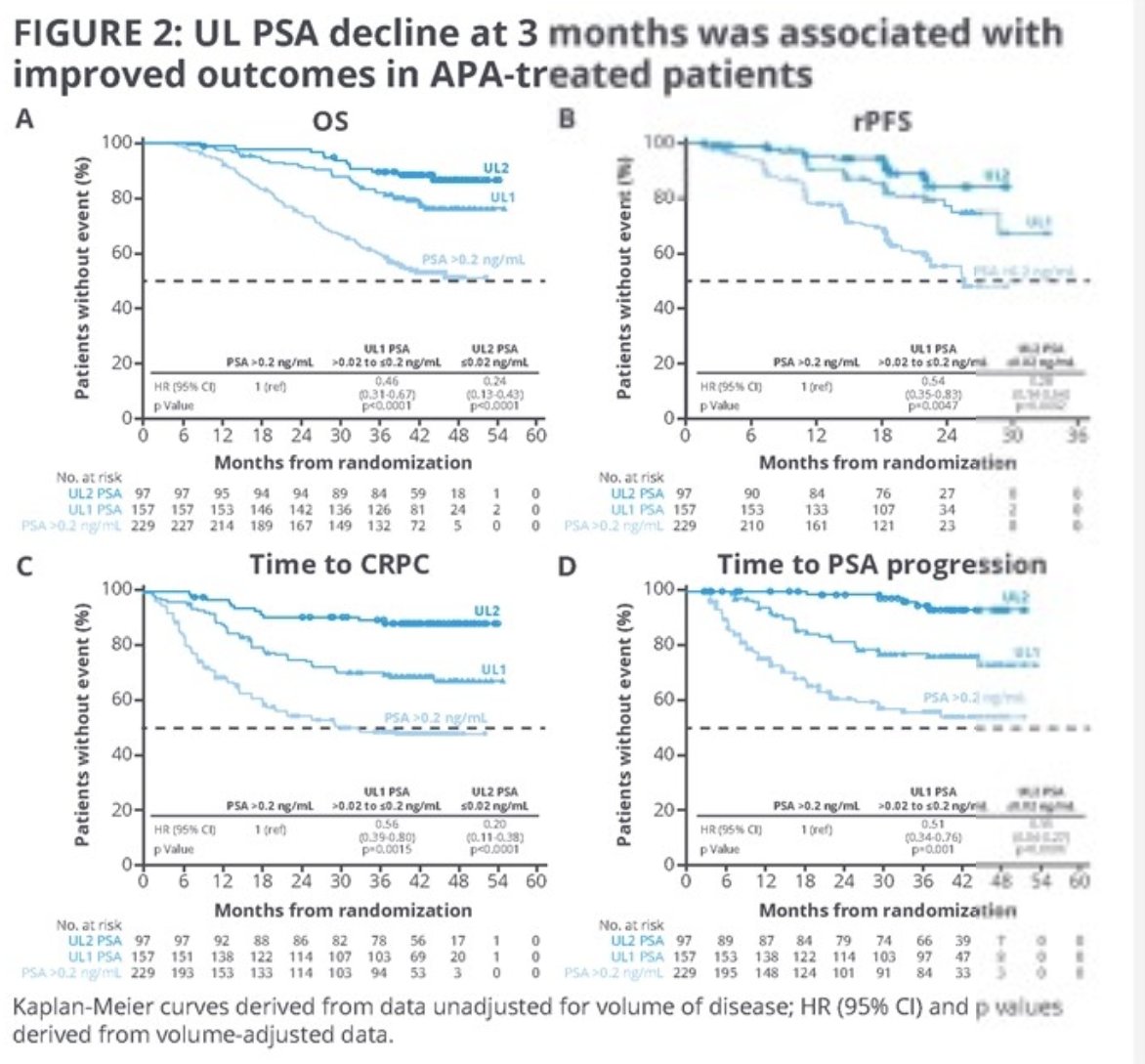
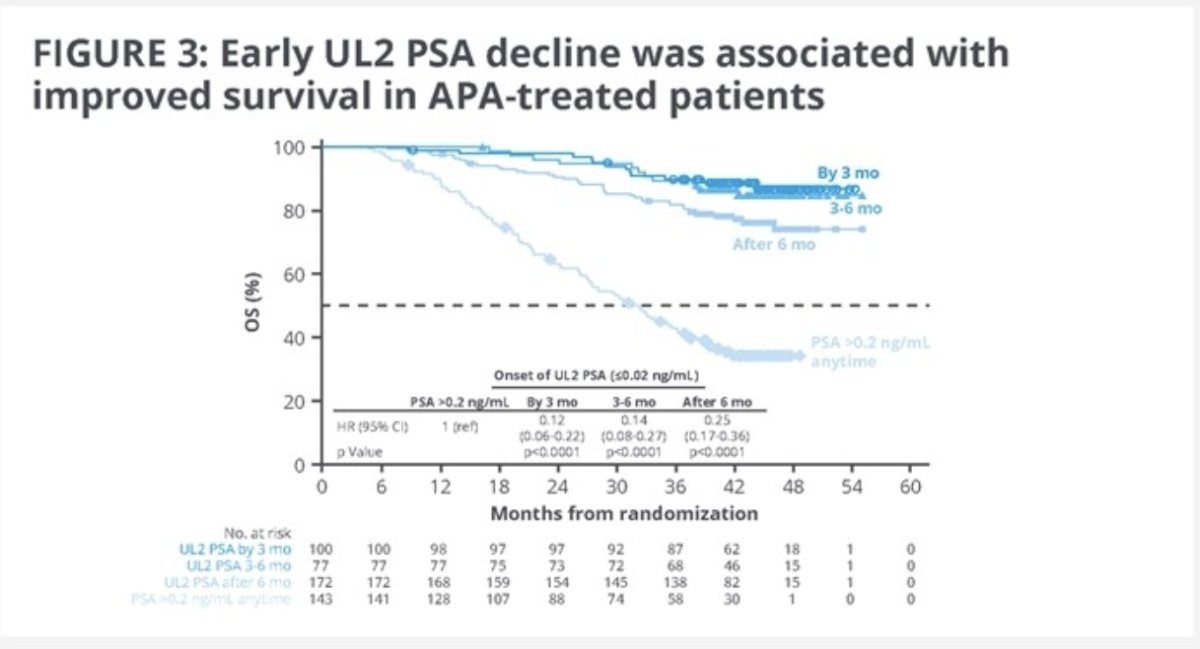
Importantly, the timing of an UL1 or UL2 PSA response appeared to be of clinical significance. At 42 months follow-up post landmark, the survival rate was 89% for those who achieved a UL2 PSA response within 3 months, compared to 81% for those who achieved it after 3 months (34% for those not achieving a UL2 PSA response). Similar survival rate patterns were observed when UL2 response was achieved before or after 6 months (89% versus 77%).
The survival rate at 42 months with UL1, UL2, or none achieved at any time were 59%, 92%, and 33%, respectively.
Presented by: Axel S. Merseburger, MD, PhD, Chairman, Department of Urology, University Hospital Schleswig-Holstein, Lubeck, Germany
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Chowdhury S, Bjartell A, Agarwal N, et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann Oncol. 2023;34(5):477-85.


