(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Hoda Abdel-Aty presented the results of an analysis evaluating prostate-specific membrane antigen positron emission tomography (PSMA PET)-directed clinical outcomes in patients with metastatic hormone-sensitive prostate cancer (mHSPC).
Over the past several years, we have witnessed an increased uptake of PSMA-PET/CT for the staging of patients in the initial staging and biochemically recurrent settings, given the improved performance characteristics of this imaging modality compared to conventional imaging.1 The use of PSMA-PET/CT in this setting has been associated with changes in the management plans, with 64% of patients in the CONDOR trial having a change in the intended disease management plan.2 However, to date, it remains unknown whether the improved sensitivity of this modality and associated PSMA-PET/CT-directed changes in the management plan lead to improved oncologic outcomes. The objective of this study was to explore the first response and progression/relapse patterns in newly diagnosed PSMA PET-detected mHSPC and correlated PET-directed staging with clinical outcomes.
To this end, the investigators retrospectively evaluated 184 patients with baseline PSMA PET scans. Patients were sub-grouped into oligometastatic or polymetastatic disease utilising the STAMPEDE2 trial definition. Data on patient demographics, treatment, and imaging findings were collected. Treatment response was assessed using PSA nadir and radiological imaging at six months from PSA nadir. Disease progression/relapse was assessed using PCWG3, RECIST 1.1, and PERCIST 1.0 criteria. The median time to radiographic progression and radiographic progression-free survival (rPFS) rates were estimated using Kaplan Meier estimates. 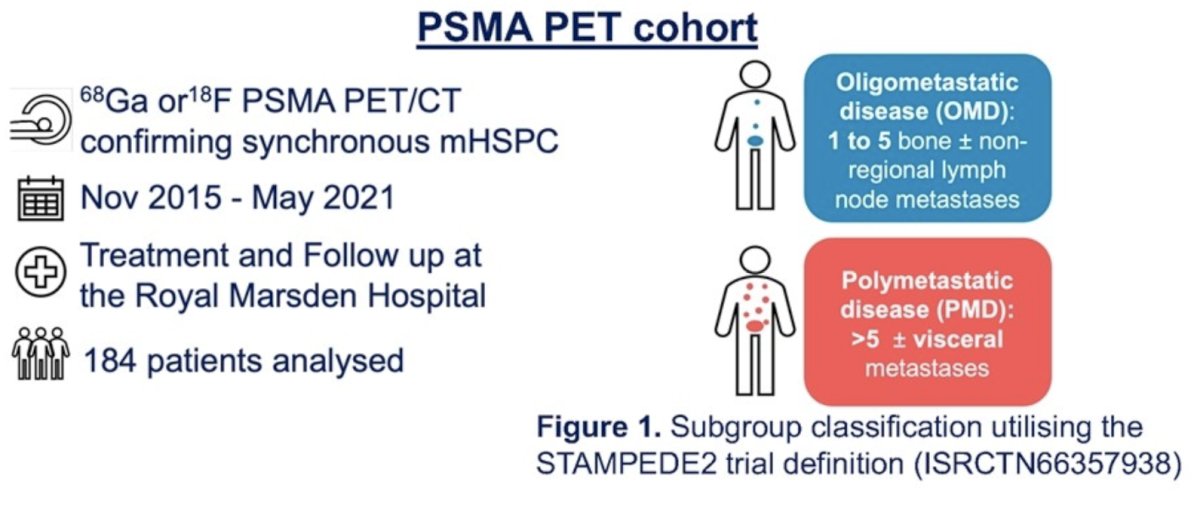
The median patient age was 69 years. All patients received ADT, with 161/184 (88%) having received additional systemic therapies.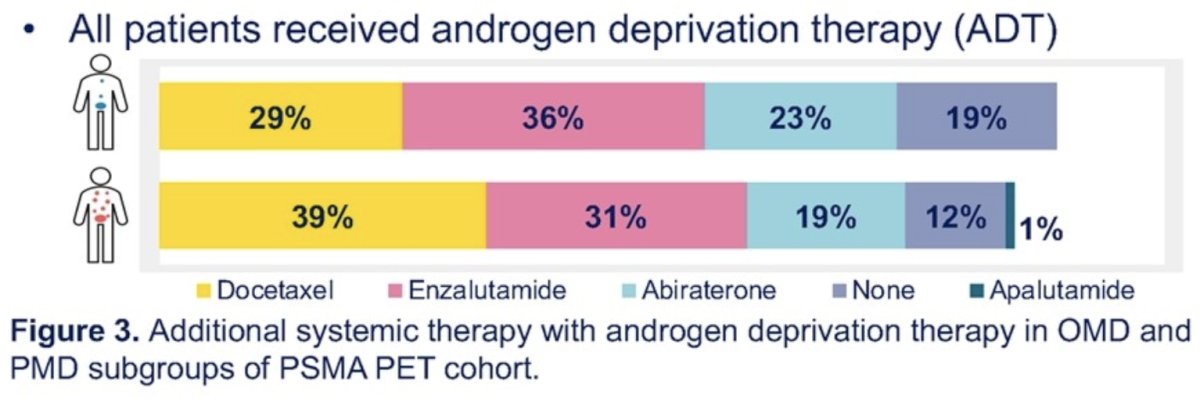
Oligometastatic and polymetastatic disease was present in 54% and 46% of patients, respectively. Prostate radiotherapy was administered to 77% and 55% of oligometastatic and polymetastatic disease patients, respectively.
The median time to PSA nadir in the two groups were 10 (range: 1 to 29) and 8 months (range: 1 to 36 months), respectively.
Response images were available for 35% and 46% of oligo- and polymetastatic patients, respectively. The median times to radiological response were 10 months (range: 2 to 58) and 9 months (range: 2 to 36), respectively. Radiological progression in oligometastatic patients most frequently occurred with new sites of disease (73%). The median rPFS was 53 months (95% CI: 40 - undefined) in oligometastatic patients and 35 months (95% CI: 22 - 43) in polymetastatic patients.
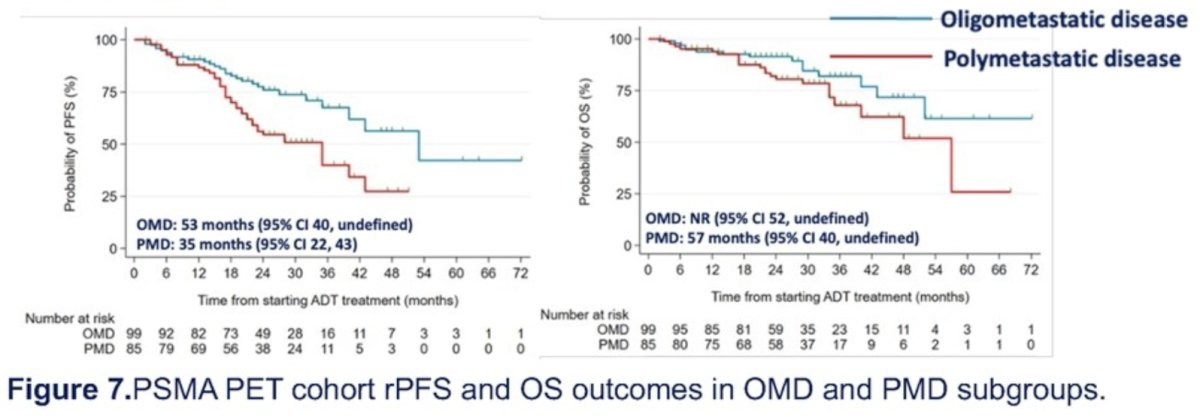
Dr. Abdel-Aty concluded that PSMA PET-guided outcomes were similar to series with conventional staging, suggesting that PET-directed therapy choices might not significantly influence time to progression. This will be further investigated in the forthcoming STAMPEDE2 trial.
Presented by: Hoda Abdel-Aty, MBChB MRCP FRCR MSc(Oncology), Clinical Research Fellow in Uro-Oncology, The Institute of Cancer Research, London, UK
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.


