(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a prostate cancer abstracts poster session. Dr. Juan Gómez Rivas presented results of the PIONEER study, the European network of excellence for big data in prostate cancer evaluating real-world trends and outcomes for European patients with metastatic hormone-sensitive prostate cancer (mHSPC).
Since 2015, we have witnessed the emergence of numerous trials demonstrating an overall survival benefit to early treatment intensification for mHSPC patients. However, despite the availability of level one evidence informing guideline changes, the clinical implementation of such recommendations has classically been poor. As such, ‘real world’ data is important to evaluate the implementation of such recommendations in real world practice. The aim of this study was to describe demographics, clinical characteristics, treatment patterns, and clinical outcomes of a large multicenter cohort of patients with mHSPC using real world data under the PIONEER project.
Data for mHSPC patients across a distributed network of observational databases were collected. Male patients without prior orchiectomy with mHSPC were enrolled in Cohort 1, while Cohort 2 was defined as the start of ADT as a surrogate definition of mHSPC disease, both in the metachronous and synchronous disease settings.
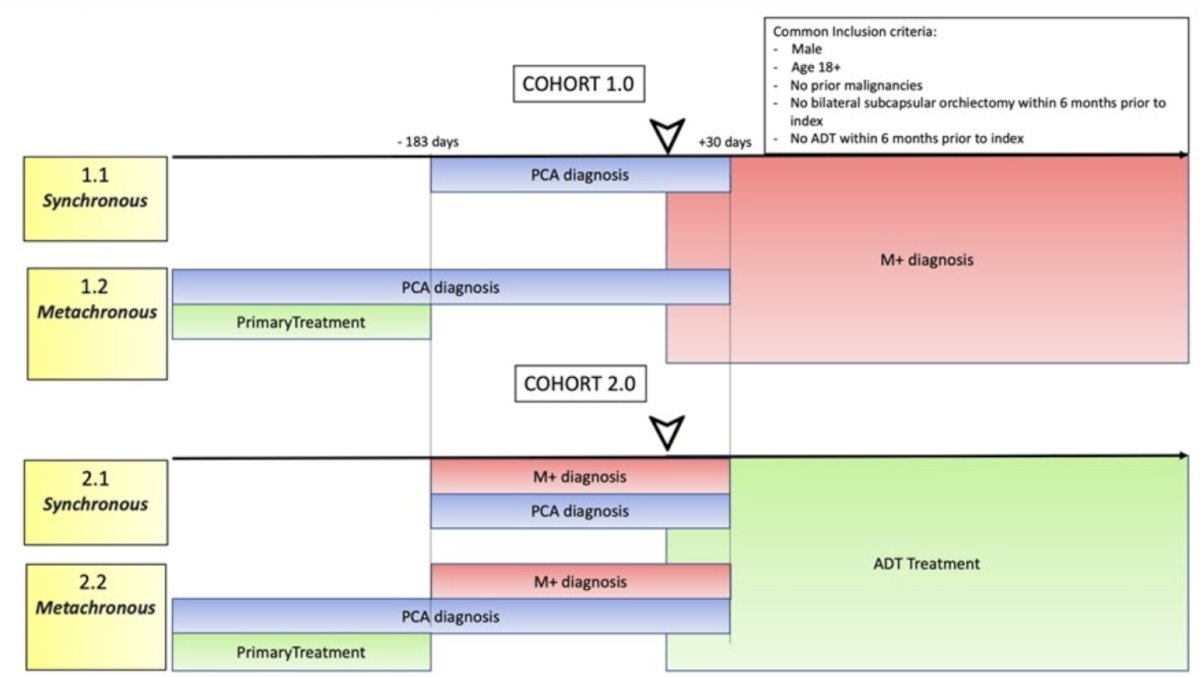
Overall, 94,261 mHSPC patients were included of whom 77,123 (81.8%) received treatment. 28% of diagnosed mHSPC patients were not on ADT monotherapy. 54% of patients were older than 70 years of age, which is a higher proportion than that observed in available randomized controlled trials. Among patients in cohort 2, 95.2% had synchronous (i.e., de novo) disease, whereas 4.8% had metachronous (i.e., recurrent) presentations. Most of the patients were treated with ADT alone.
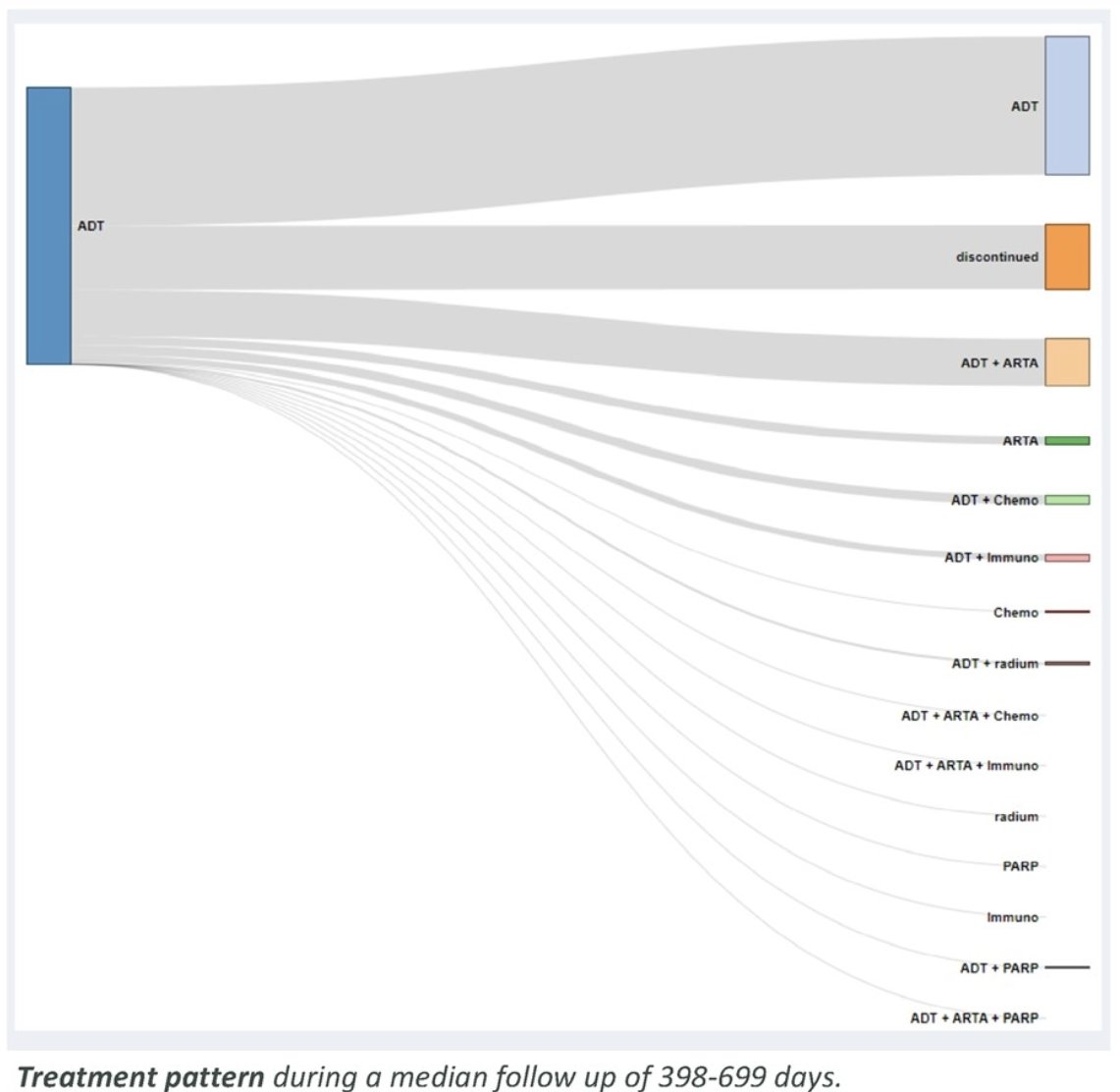
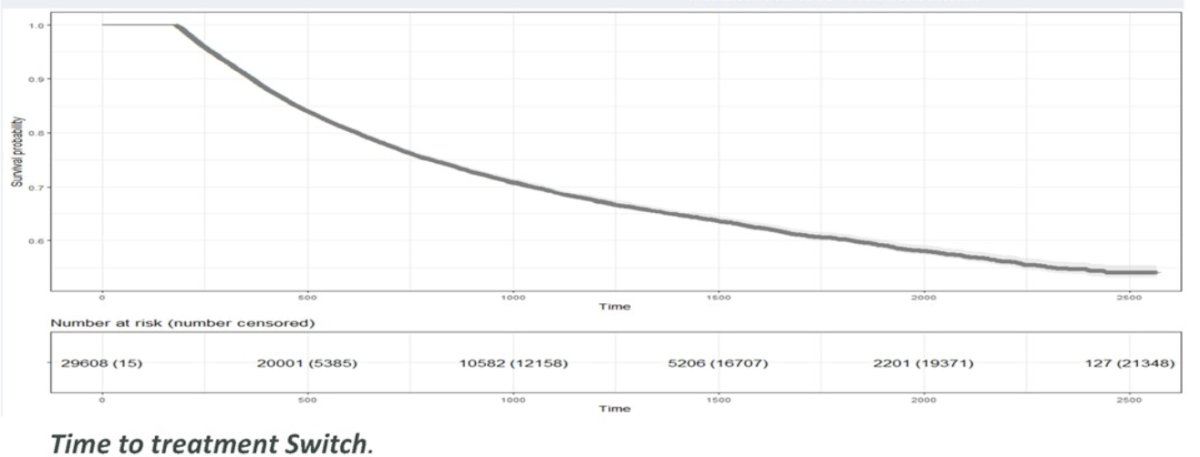
At a median follow-up range of 398 to 699 days in the metachronous setting, 22% of patients discontinued the treatment. Clinical outcomes such as time to admission to the hospital or emergency department, adverse events and death increased with time, but were noticeably more common among patients with synchronous disease.
Dr. Rivas concluded that this is the largest study evaluating real world outcomes of mHSPC patients in Europe. The prostate cancer treatment landscape is constantly evolving, so it is important to understand the behavior of the disease in the real-world setting, in order to fill gaps or create new questions to generate further evidence.
Presented by: Juan Gómez Rivas, MD, PhD, Clínico San Carlos University Hospital, Madrid, Spain
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


