The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Kim Chi discussing the final results of the MAGNITUDE trial assessing niraparib with abiraterone acetate plus prednisone as first-line therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) and HRR gene alterations.
Patients with mCRPC and HRR gene alterations, especially those with BRCA 1/2 alterations, have poor outcomes. In the phase 3 MAGNITUDE study, niraparib + abiraterone acetate + prednisone significantly improved radiographic progression-free survival in BRCA-mutant (BRCA+) patients (HR 0.53, 95% CI 0.36-0.79) [1]. At the 2023 ESMO annual meeting, Dr. Chi and colleagues presented the final analysis of MAGNITUDE, a phase 3 study with the largest population of first-line BRCA+ mCRPC patients, reporting mature overall survival (OS) data and prespecified multivariable analysis for OS, addressing baseline imbalances.
Eligible patients (n = 423) with HRR+ mCRPC were randomized 1:1 to niraparib + abiraterone acetate + prednisone (n = 212) or placebo + abiraterone acetate + prednisone (n = 211) as first-line therapy: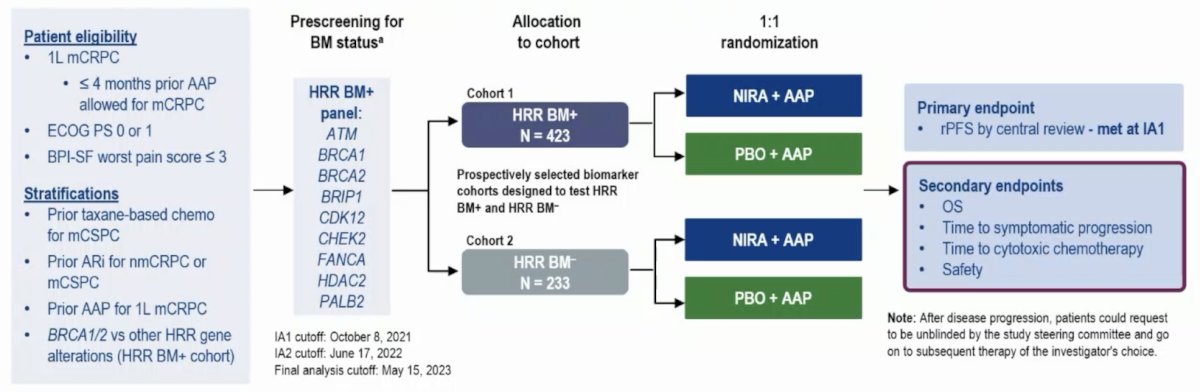
At the final analysis, secondary endpoints of OS and time to cytotoxic chemotherapy were formally assessed. Updates to time to symptomatic progression and patient-reported outcomes in BRCA+ patients and to safety for all HRR+ patients are also reported.
At the final analysis, 225 BRCA+ patients were evaluated, of which 113 patients received niraparib + abiraterone acetate + prednisone. Assessing the baseline characteristics, the placebo + abiraterone acetate + prednisone arm had more favorable characteristics, which impacted the comparison to niraparib + abiraterone acetate + prednisone: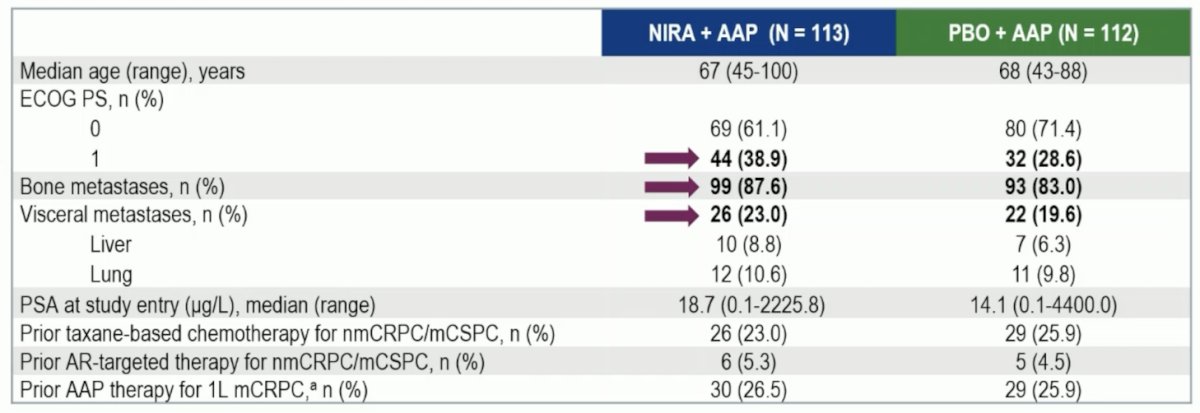
The median follow-up of MAGNITUDE was 35.9 months. In the niraparib + abiraterone acetate + prednisone and placebo + abiraterone acetate + prednisone arms, 70% and 86% of patients received subsequent life-prolonging therapy. OS favored niraparib + abiraterone acetate + prednisone over placebo + abiraterone acetate + prednisone (HR 0.788, 95% CI 0.554-1.120):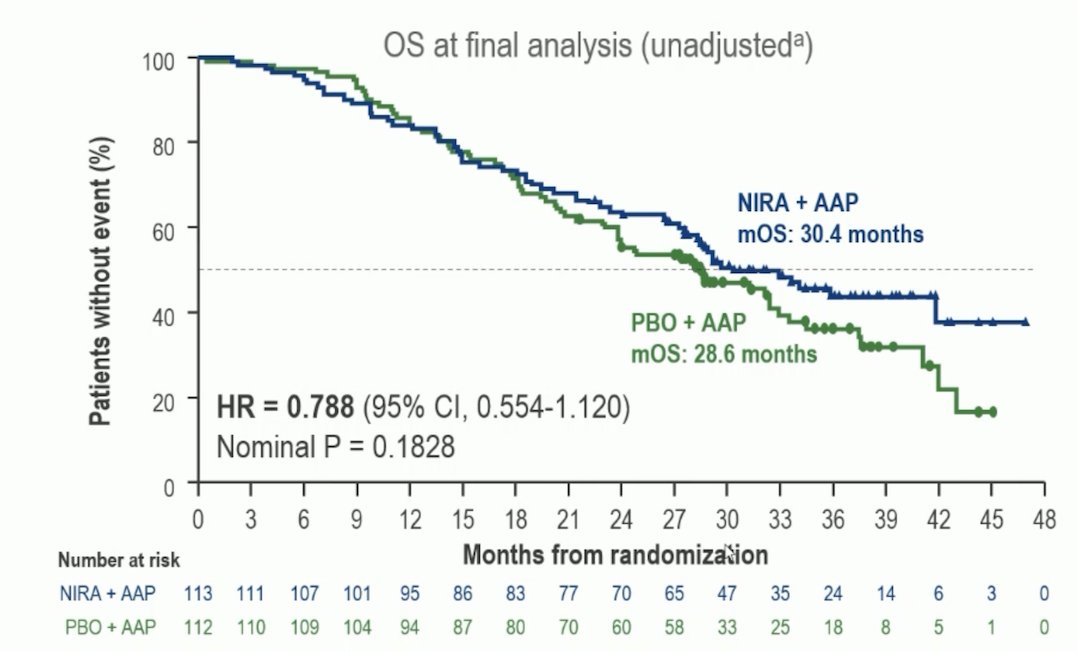
A prespecified multivariable analysis adjusting for baseline imbalances showed an OS benefit favoring niraparib + abiraterone acetate + prednisone (HR 0.663, 95% CI 0.464-0.947). In the niraparib + abiraterone acetate + prednisone arm 53.1% of patients discontinued treatment due to disease progression compared to 78.8% in the placebo + abiraterone acetate + prednisone arm. The subsequent life prolonging therapies are highlighted: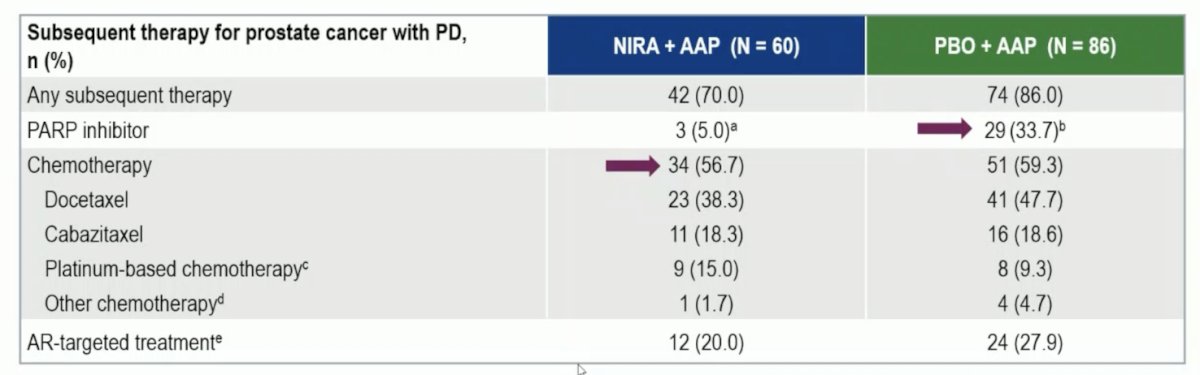
Continued improvement in time to symptomatic progression and a clinically meaningful improvement in time to cytotoxic chemotherapy were observed with niraparib + abiraterone acetate + prednisone: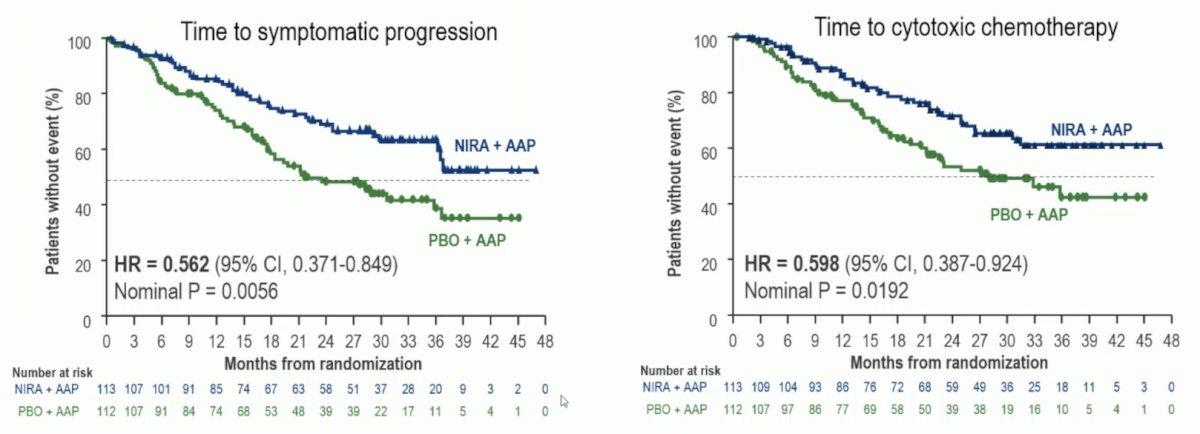
No new safety signals were observed with additional treatment exposure. Pulmonary embolism occurred in 4.7% and 1.4% of patients in niraparib + abiraterone acetate + prednisone and placebo + abiraterone acetate + prednisone arms, with no cases of myelodysplastic syndrome or acute myeloid leukemia in the niraparib + abiraterone acetate + prednisone arm. Transfusion rates were 27.3% in the niraparib + abiraterone acetate + prednisone arm compared to 5.2% in the placebo + abiraterone acetate + prednisone arm
Dr. Chi concluded his presentation discussing the final results of the MAGNITUDE trial assessing niraparib with abiraterone acetate plus prednisone as first-line therapy in patients with mCRPC and HRR gene alterations with the following take-home points:
- With 11.1 months of additional follow-up from the second interim analysis and 19.2 months from the primary analysis, OS favored niraparib + abiraterone acetate + prednisone for patients with BRCA+ mCRPC
- A prespecified multivariable analysis adjusting for prognostic factors supported a stronger OS benefit for niraparib + abiraterone acetate + prednisone
- Niraparib + abiraterone acetate + prednisone led to improvements in time to symptomatic progression, time to cytotoxic chemotherapy, and patient-reported outcomes
- Safety remained consistent with the known safety profile of niraparib and other PARP inhibitors
- The final analysis of MAGNITUDE supports the positive benefit-risk profile supports first-line niraparib + abiraterone acetate + prednisone as a new standard of care for patients with BRCA+ mCRPC
Presented by: Kim Chi, British MD, FRCP, Columbia Cancer Agency, Vancouver, British Columbia, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
Reference:


