(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Emilio Giunta discussing the results of the phase II clinical trial assessing 177Lu-PSMA-617 in the pre- and post-taxane mCRPC setting. 177Lu-PSMA-617 prolonged progression-free survival (PFS) and overall survival (OS) versus standard of care in mCRPC patients, as shown in the phase III VISION trial [1]. Of note, as part of the trial design, patients in VISION already received taxane-based chemotherapy. At the 2023 ESMO annual congress, Dr. Giunta and colleagues reported their analysis from a phase II trial of 177Lu-PSMA-617 administered in the pre- and post-taxane mCRPC settings.
In the IRST-185.03 study, which is an open-label, single-center, phase 2 prospective trial, 145 mCRPC patients were enrolled between April 2017 and October 2022. There were 142 patients that received up to 6 cycles of 177Lu-PSMA-617 every 6 weeks, with a mean dose of 17.5 GBq (up to 33 GBq of total dose). The best biochemical response, defined as ≥50% PSA reduction from baseline, was the main clinical endpoint of the trial, while PFS, OS, and safety were the main secondary endpoints.
Among 142 evaluable patients, 100 patients received at least one cycle of taxane-based chemotherapy for castration-sensitive and castration-resistant disease (post-taxane group), whilst 42 were taxane-naïve (pre-taxane group). Baseline clinical characteristics of the two groups were balanced in terms of age, Gleason score, and previous ARSI, except for a higher proportion of ECOG performance status 1-2 patients in the post- versus pre-taxane group (42% and 16.7%, respectively):
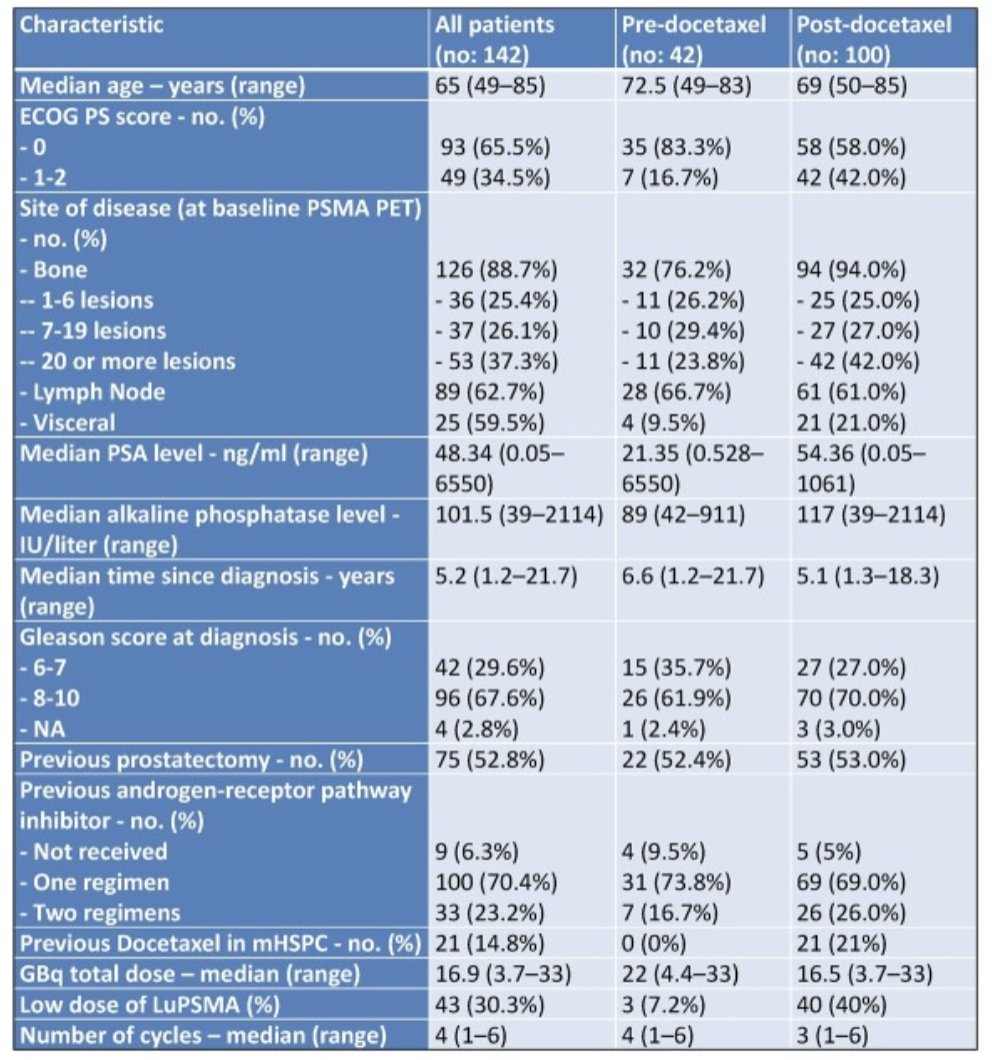
At the first interim analysis, after a median follow-up of 28.8 months, best biochemical response was obtained in 23 (54.8%) and 35 (35%) patients in the pre- and post-taxane groups, respectively. Additionally, median PFS was 8.5 and 6.0 months in the pre- and post-taxane groups, respectively:
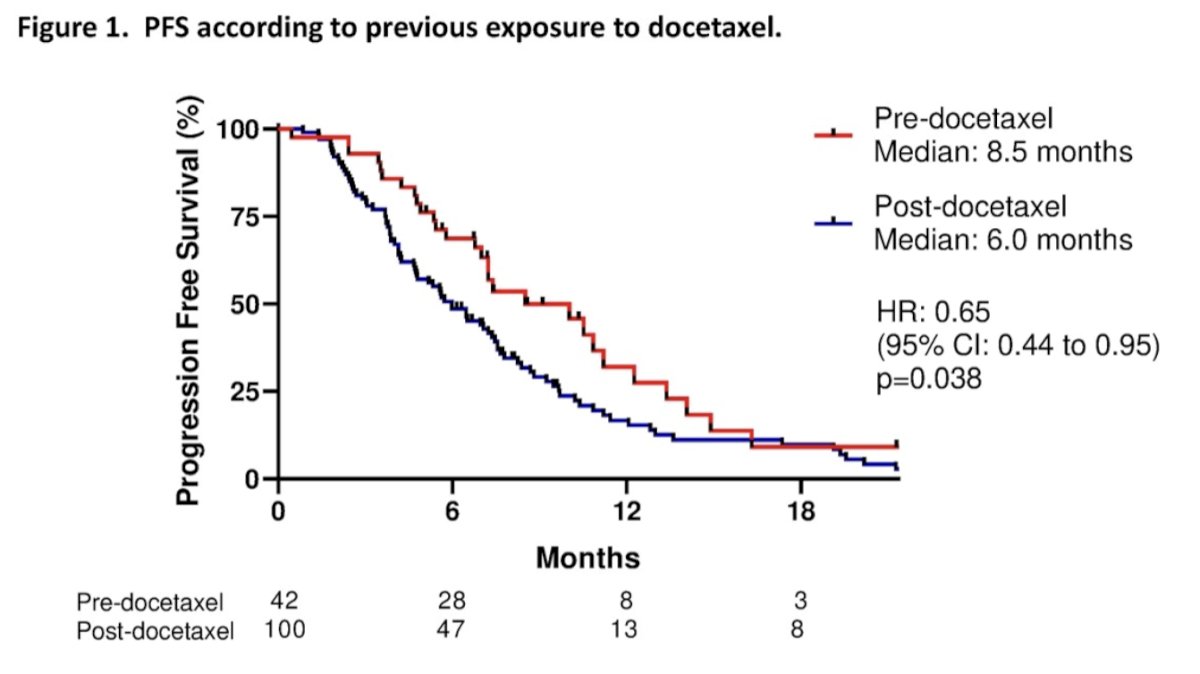
Median OS was 35.1 and 12.6 months in the pre- and post-taxane groups, respectively:

The main clinical outcomes according to previous exposure to docetaxel are as follows:
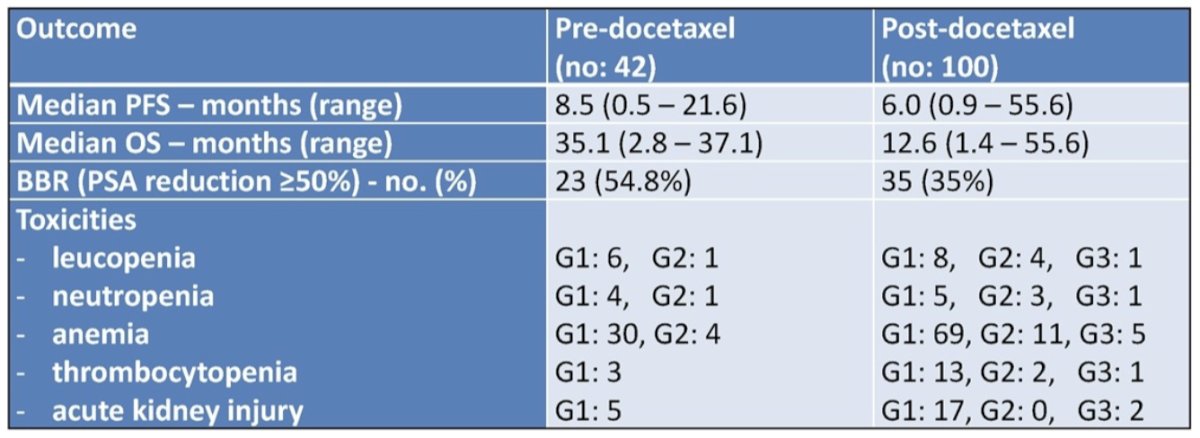
Regarding safety, anemia was the most common adverse event (pre vs post docetaxel):
No grade 3 adverse events were recorded in the pre-taxane group.
Dr. Giunta concluded his presentation by discussing the results of the phase II clinical trial assessing 177Lu-PSMA-617 in the pre- and post-taxane mCRPC setting by highlighting that 177Lu-PSMA-617 is effective and safe in taxane-naïve mCRPC patients, therefore it could represent a valid therapeutic choice in the pre-docetaxel mCRPC setting.
Presented by: Emilio F. Giunta, University of Campania Vanvitelli, Napoli, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:


