(UroToday.com) The 2023 ESMO annual meeting included a Presidential session, featuring a discussant presentation by Dr. Christopher Sweeney discussing the PSMAfore phase 3 trial. Dr. Sweeney started by highlighting that there are several clear strengths of the PSMAfore trial:
- It was a well-powered randomized controlled phase 3 trial
- There was clear documentation of activity: cancer regression, delay in progression, quality of life, and pain control
- The data is set for patients that are not fit for docetaxel
- Allowed crossover in the control group
However, there are some limitations:
- rPFS is a modest endpoint compared to overall survival
- Hormone switch is a “weak” control – although in the rapidly changing landscape of advanced prostate cancer, these trials are designed years prior
- There is no defining optimal dosing – what is optimal PSMA uptake? Should we hold treatment if PSMA uptake resolves like in the recently presented ENZA-p trial?
- Long term safety data is needed, especially with longer overall survival and use of PARP inhibitors and/or platinum chemotherapy
As follows is Dr. Sweeney’s table placing LuPSMA CRPC data into context:
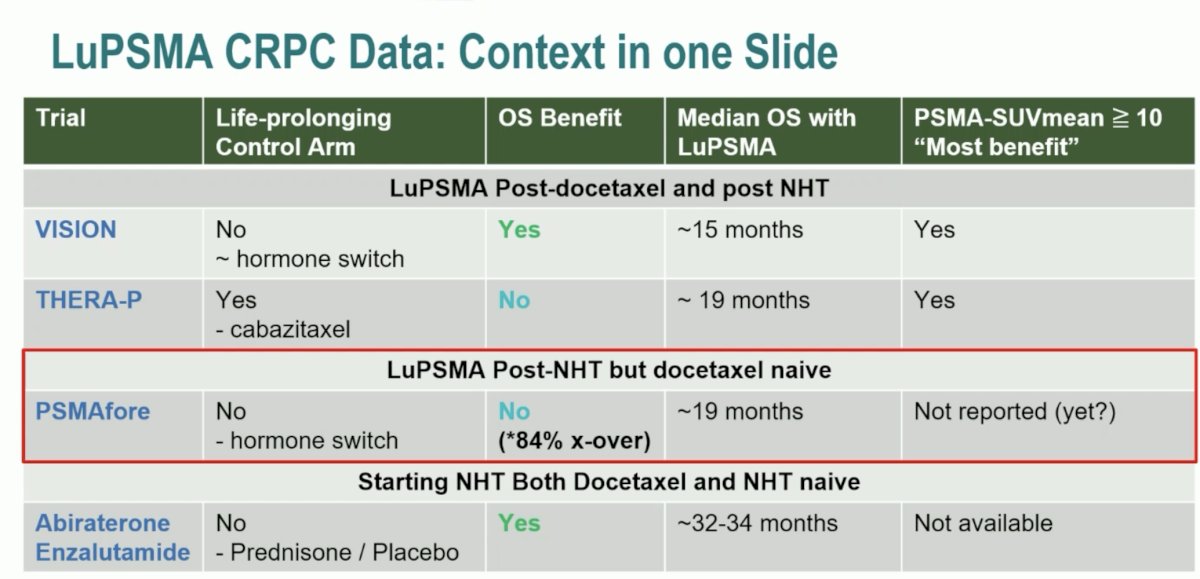
The bottom line is that LuPSMA is an effective therapy and prolongs overall survival. It does not replace any existing CRPC options but is one option and one chess piece on the chessboard. Additionally, PSMAfore does not tell us when best to use LuPSMA, but it does provide data for the use prior to docetaxel. We need to define the best first line mCRPC option for individual patients, given that many patients are unable to go from 1st to 2nd to 3rd line of therapy and 15% of PSMAfore patients did not cross over early. Ultimately, our goal is to use as many life prolonging therapies as possible. Putting LuPSMA in mCRPC into further context, Dr. Sweeney notes that PSMAfore is one-data point in the “heat-map” of mCRPC treatment:
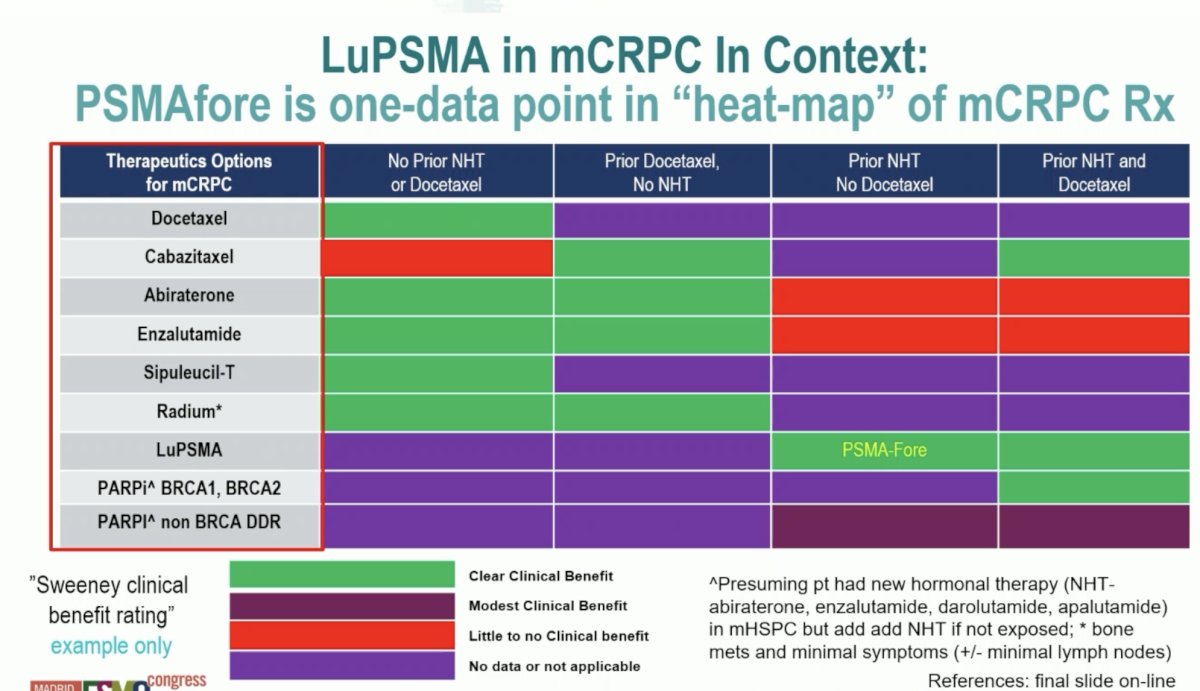
Unfortunately, divergent drug development strategies lead to divergent messages and ultimately confusion with regards to selecting first line treatment for mCRPC. There are several key angles highlighted by Dr. Sweeney:
- Industry, academia, and regulators/payers: need to develop effective therapies, decrease burden of cancer, and assess health economics versus other therapies
- Broad patient population: often regulatory framework, minimize risk of missing effective treatment, risk of futile toxic therapy and missing active treatment
- More precision: often sub-studies/academic explorations, less risk of futile therapy, risk of not treating some who may benefit

Dr. Sweeney concluded his presentation discussing the PSMAfore phase 3 trial with two personalized treatment principles for patients with metastatic prostate cancer:
- Principle #1: All patients with metastatic hormone sensitive prostate cancer should be offered the most effective hormonal therapy available
- Principle #2: Deploy precision medicine for first-line mCRPC therapy
- PSMA PET/CT to profile the patient
- Assess suitability for docetaxel (or cabazitaxel if docetaxel was received in mHSPC)
- Obtain tumor exome profiling: BRCA1, BRCA2, other DDR variants, MSI, p53, RB1, PTEN
- Collate the data to develop an individual treatment plan with the greatest chance of benefit as the first line therapy for each patient

Presented by: Christopher Sweeney, MBBS, University of Adelaide, Adelaide, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
Related Content:
ESMO 2023: PSMAfore Phase 3 Trial of [177Lu]Lu-PSMA-617 in Taxane-Naive Patients with Metastatic Castration Resistant Prostate Cancer


