(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a ‘Dissecting metastatic castration-sensitive prostate cancer (mCSPC) for optimal patient benefit’ session. Dr. Gerhardt Attard discussed how best to integrate biology into the treatment of mCSPC patients.
Currently, ADT plus androgen receptor pathway inhibitors (ARPIs) remain the backbone of treatment for all fit patients. Prostate radiotherapy may be considered in patients with de novo CHAARTED low volume mCSPC. Additional questions remain in this setting:
- Who should get additional treatment?
- What form should this take?
- Who should be considered for de-escalation?
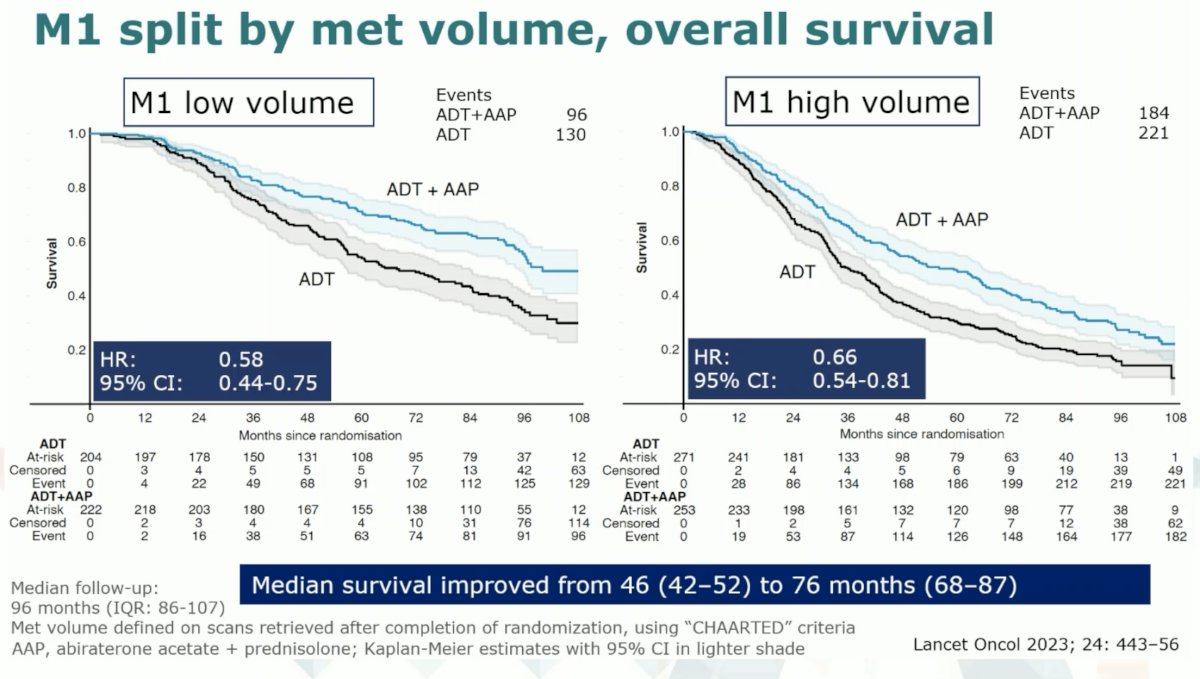
The latest update of the STAMPEDE Abiraterone data at a median follow-up of 8 years clearly demonstrates continued long-term survival benefits for early abiraterone in mCSPC patients, both with low and high-volume disease.1 This contrasts with the STAMPEDE docetaxel data where the survival advantage levels off over time.2,3
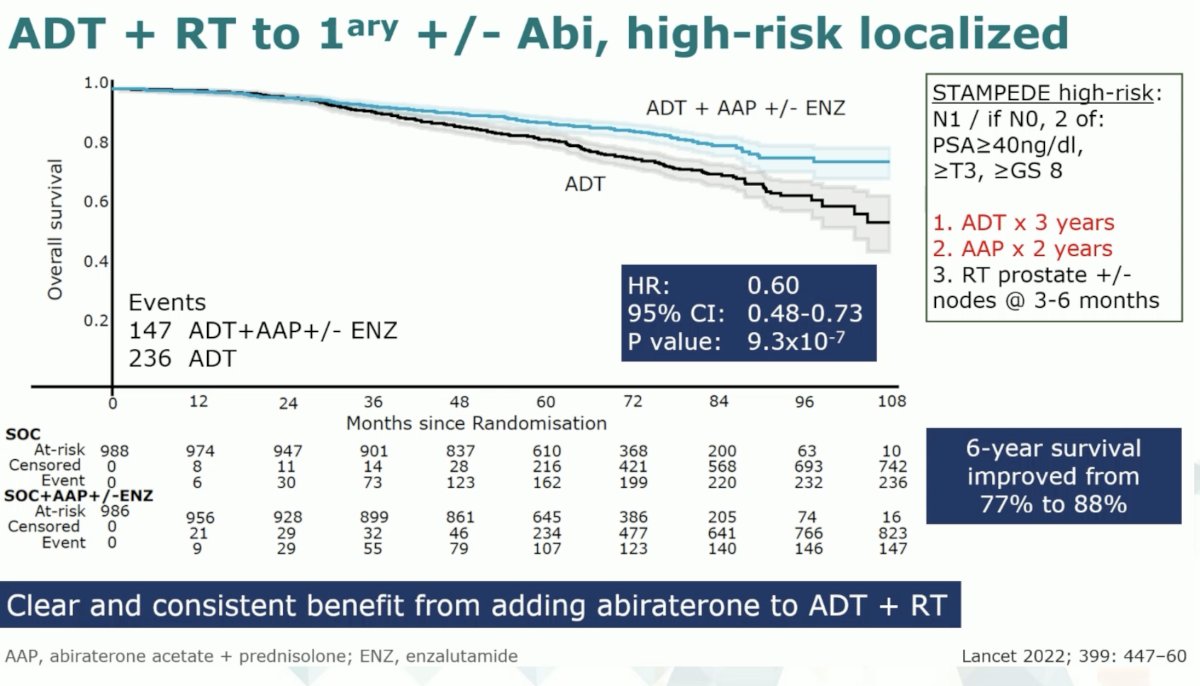
Data from the STAMPEDE platform has also demonstrated that the addition of abiraterone to ADT + radiotherapy in patients with high-risk, localized disease was associated with overall survival benefits (6-year survival: 88% versus 77%; HR: 0.60, 95% CI: 0.48 – 0.73, p<0.001).4 This cohort of patients included those with cN+ disease on conventional imaging, or if node negative then having 2+ of the following 3 features:
- PSA ≥40 ng/ml
- Gleason Score ≥8
- ≥T3 disease
Of note patients in this trial were limited to 3 years of ADT and 2 years of abiraterone, which is in contrast to the trials in the mCSPC setting, where these drugs are continued indefinitely.
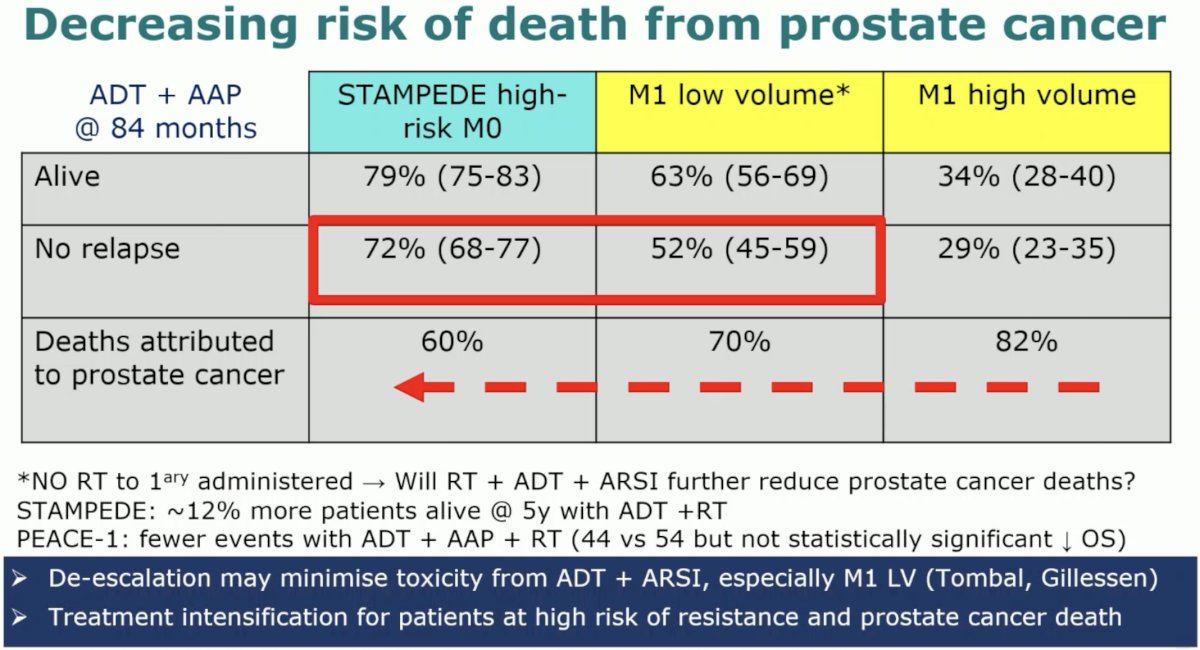
What is clear is that the more advanced the disease along a continuum from high-risk M0 to M1 low and M1 high volume, the deaths attributed to prostate cancer, among those who experienced mortality, were more likely to be attributed to prostate cancer as the disease progresses along the continuum (60 to 80%).
Results of the PEACE-1 trial demonstrate that the addition of abiraterone to doublet ADT + docetaxel improves overall survival in the de novo M1 high volume patients, but not the low volume cohort to date.5 However, whether the addition of docetaxel to ADT + abiraterone improves survival remains unknown.
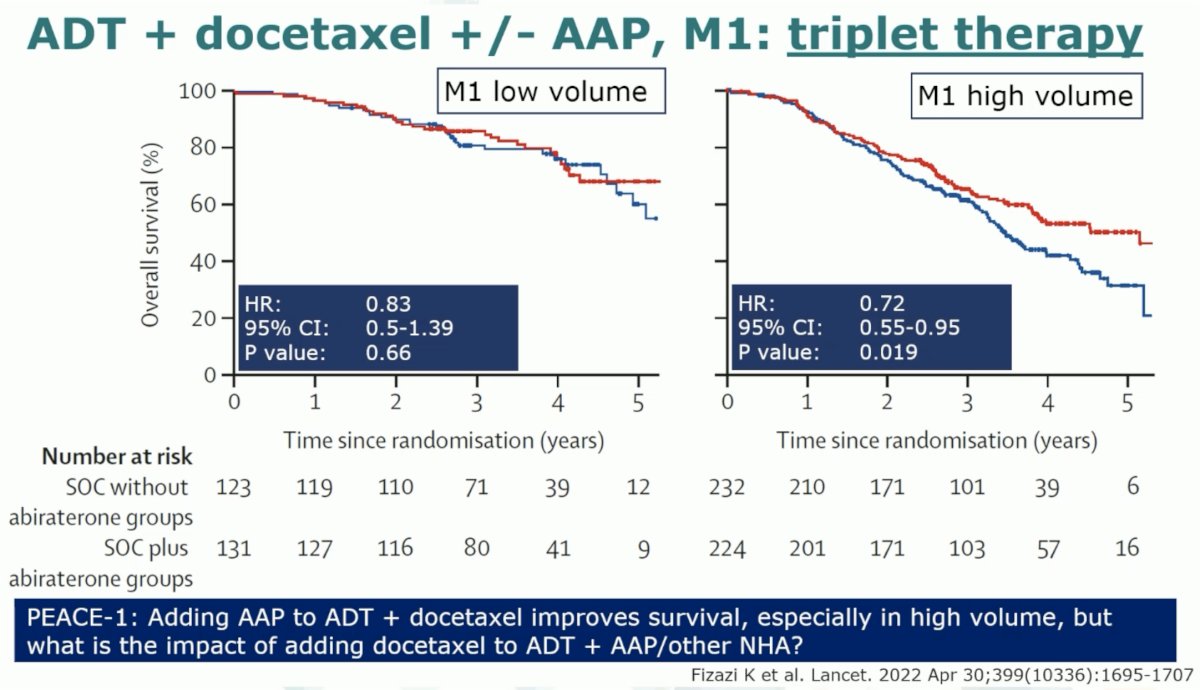
It does appear that the greatest benefit to the early addition of docetaxel is in the high volume de novo patients, particularly those with bulky disease (high volume cT4 disease).6
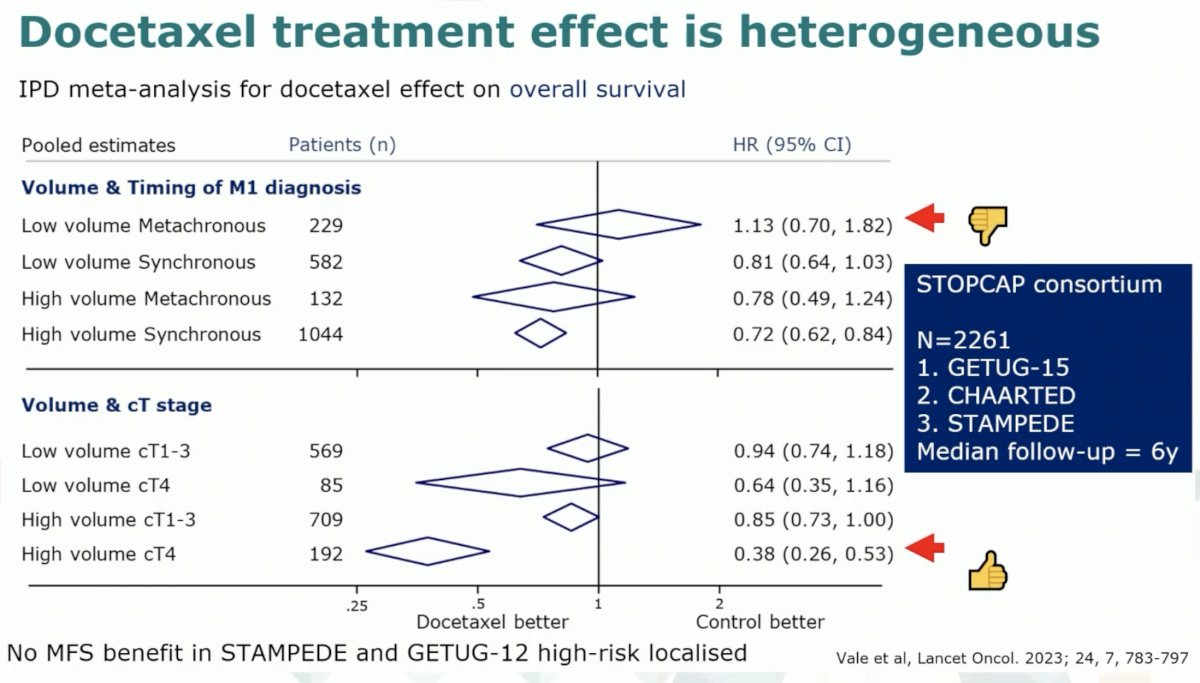
There is a cost to using docetaxel. Initially, there is a significant reduction in quality of life, with patients treated with abiraterone having considerably better quality of life outcomes that are maintained over two years. As such, it is clear that we need additional prognostic tests to determine who needs docetaxel and predictive tests to evaluate whether it will work, particularly in patients receiving ADT + an ARPI.

How can biology help in this setting? The molecular characterization of tumors linked to prospective outcome data can identify biological processes that underly aggressiveness leading to biomarkers for stratification or therapeutic targeting. This will entail:
- Use of data from large datasets with prospective follow-up
- Consortia collaborating on similar analysis protocols
- Samples from randomized trials that allow for prediction of outcomes
- Use of ‘fit for purpose’ tests that help deal with “challenging” quality tissue
Additionally, new targets/biological insights from mCRPC could be evaluated at the start of ADT.
Recent work from the STAMPEDE abiraterone trial has evaluated molecular signatures across the disease spectrum. The investigators retrieved prostate tissue specimens, namely prostate needle core biopsies, from almost 1,300 patients. These underwent H&E staining and subsequently underwent tumor area microdissection. Expression array analysis was subsequently performed evaluating ~40,000 with 59 transcriptomic signatures generated using 1.4 million probes.

The generated transcriptomic signatures (57 continuous and 2 categorical) inform on the activity of biological processes putatively important to prostate cancer. The androgen-related signatures are both correlated and ‘anti-correlated’ with ‘neuroendocrine’ signatures. Notably, immune signatures developed in other cancer types can show large inter-patient variability. Significantly, the proliferation signatures demonstrated a significant difference in the localized N0M0 state compared to more advanced states such as the M1 high volume state.
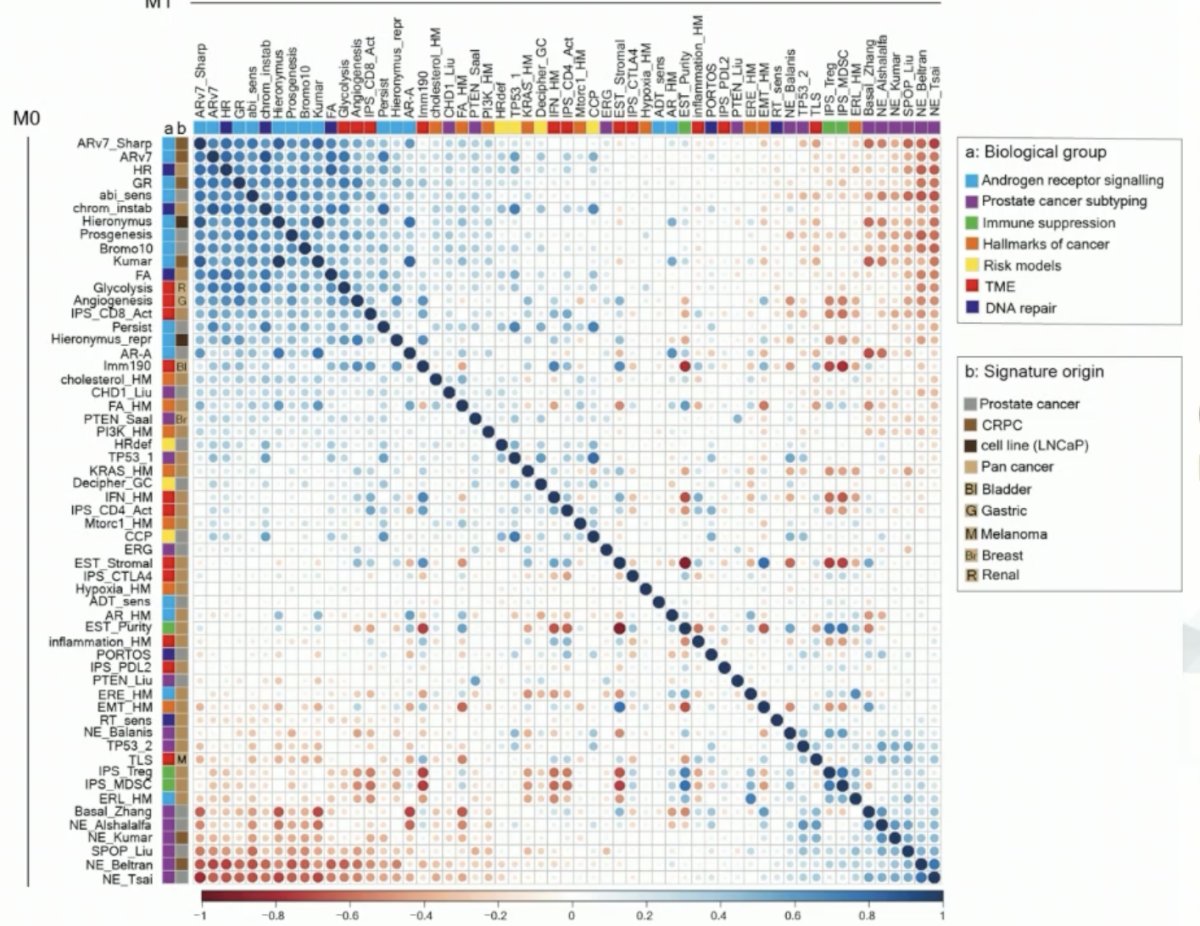
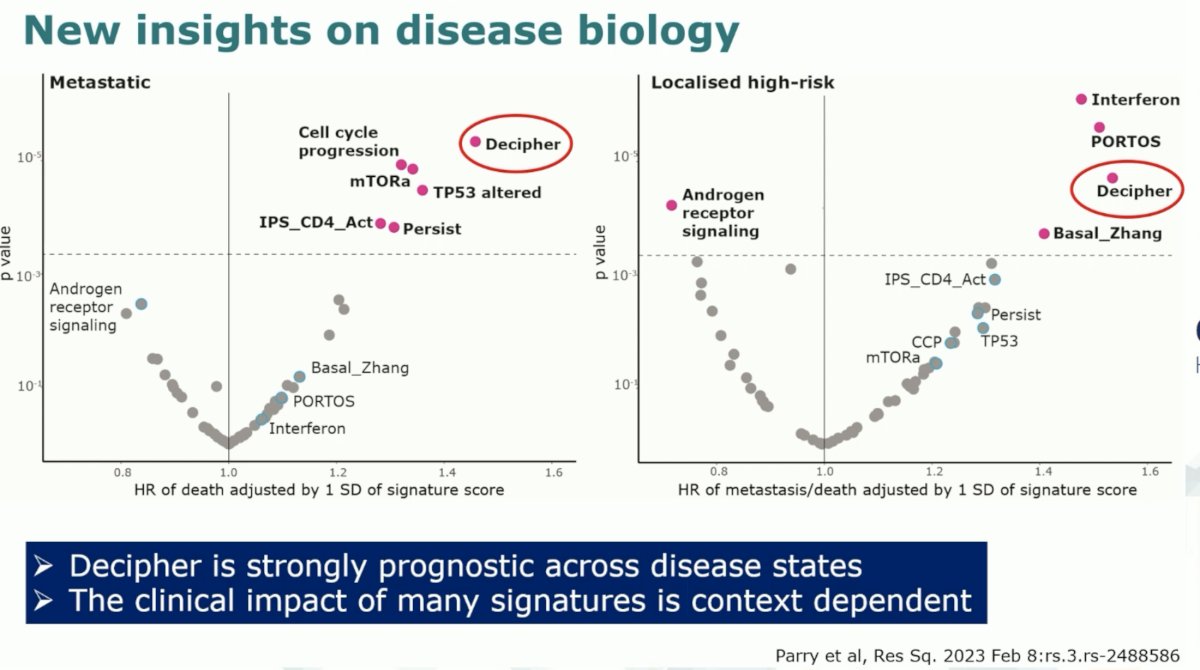
When plotting the prognostic hazard ratios for each signature across the localized, high-risk, and metastatic disease states, the investigators noted that the Decipher signature was the only one that was strongly prognostic across disease states. It is important to note that the clinical impact of many of these signatures as seen below is context (i.e., disease state) dependent.
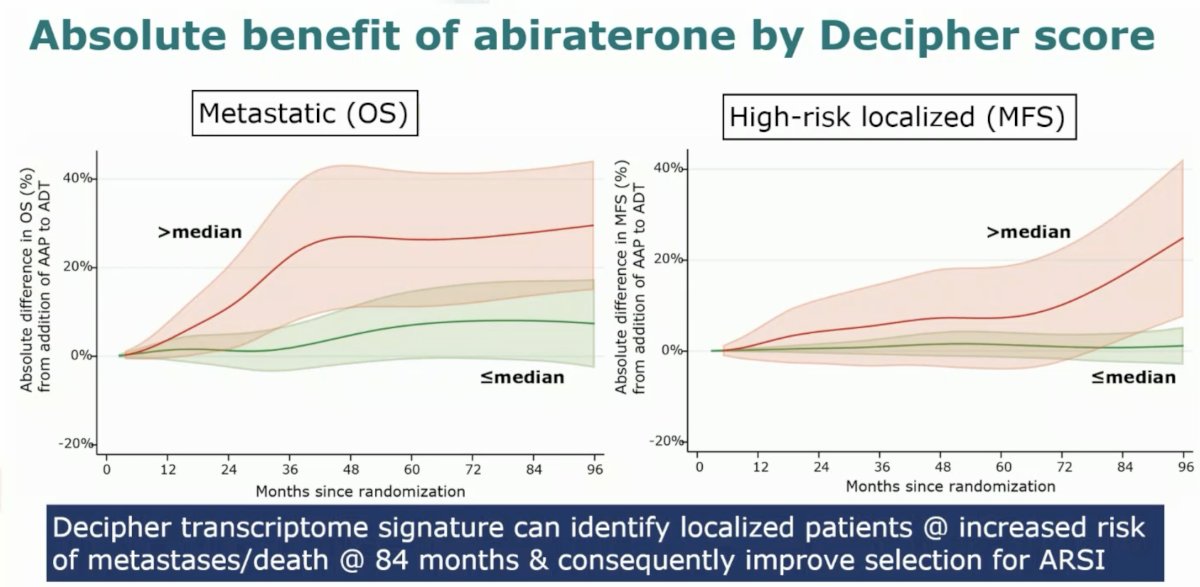
It does appear that Decipher may potentially have benefit as a predictive biomarker. Among castrate-sensitive patients in the M1 disease state, patients with a score higher than the pre-determined median derived a much higher overall survival benefit from the addition of abiraterone to ADT, compared to those with scores below the median. Similarly, among the high-risk, localized disease patients, those with a score above the median derived a significantly improved MFS benefit from the use of abiraterone with ADT and radiotherapy.
The transcriptomic signature also identified mTORa mutations are associated with worse survival outcomes. High mTORa scores are associated with negative PTEN immunohistochemistry staining and could potentially identify cancers for targeting with AKT inhibition, such as ipatasertib.
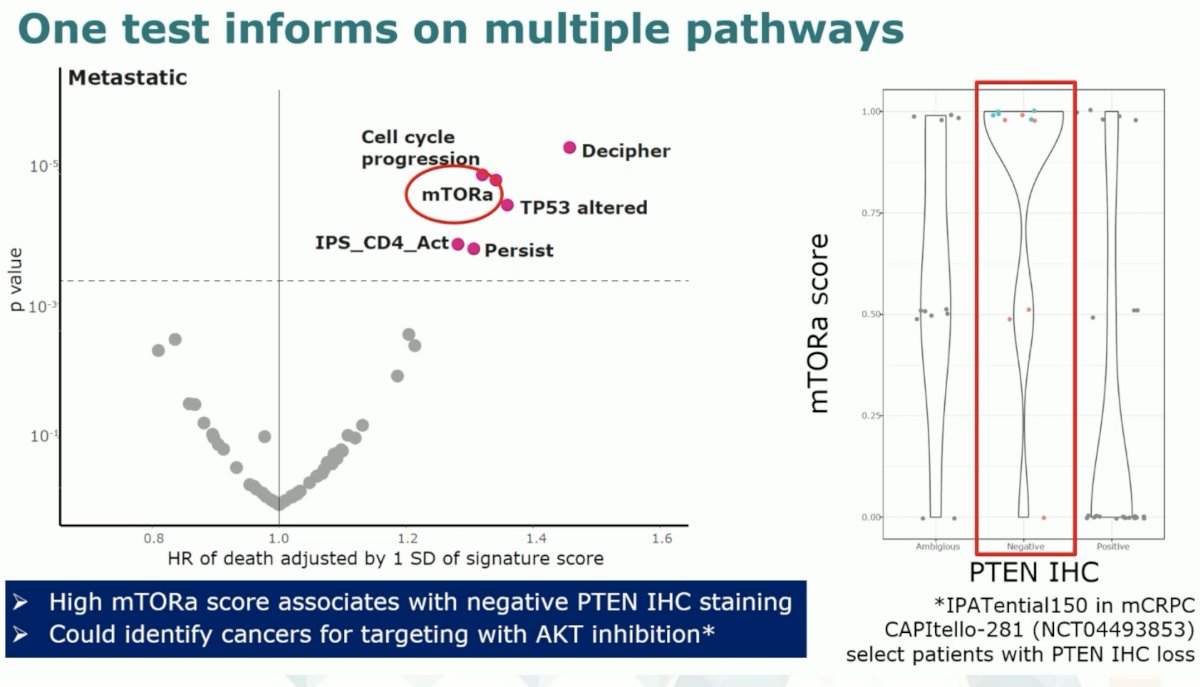
Transcriptomic profiling from the CHAARTED trial has identified subgroups that may derive a differential benefit from docetaxel.

Another emerging prognostic tool is ArteraAI which relies on multimodal artificial intelligence modeling of H&E slides to generate MMAI scores. Presented by Dr. Charles Parker during this meeting, patients with M1 low- and high-volume disease can be further stratified by the MMAI score to generate 4 quartiles with a progressively worsening risk of 5-year prostate cancer mortality.

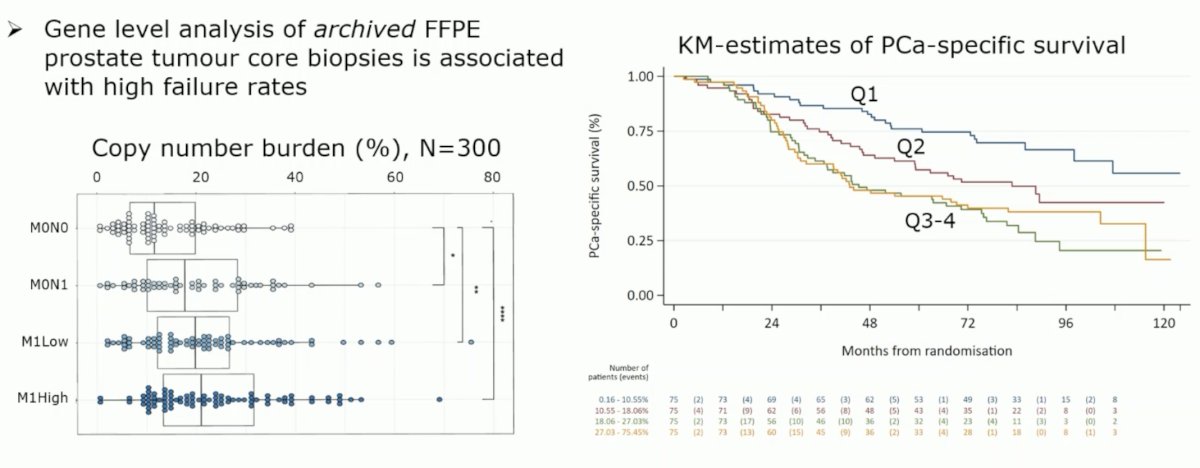
What about biology contained in DNA? One challenge is that gene level analysis of archived FFPE prostate tumor core biopsy tissue is associated with high failure rates. However, it does appear that an increasing copy number burden is associated with worse metastatic burden. Interestingly, gains appear to account for an increasing proportion of copy number changes as copy number burden increases.
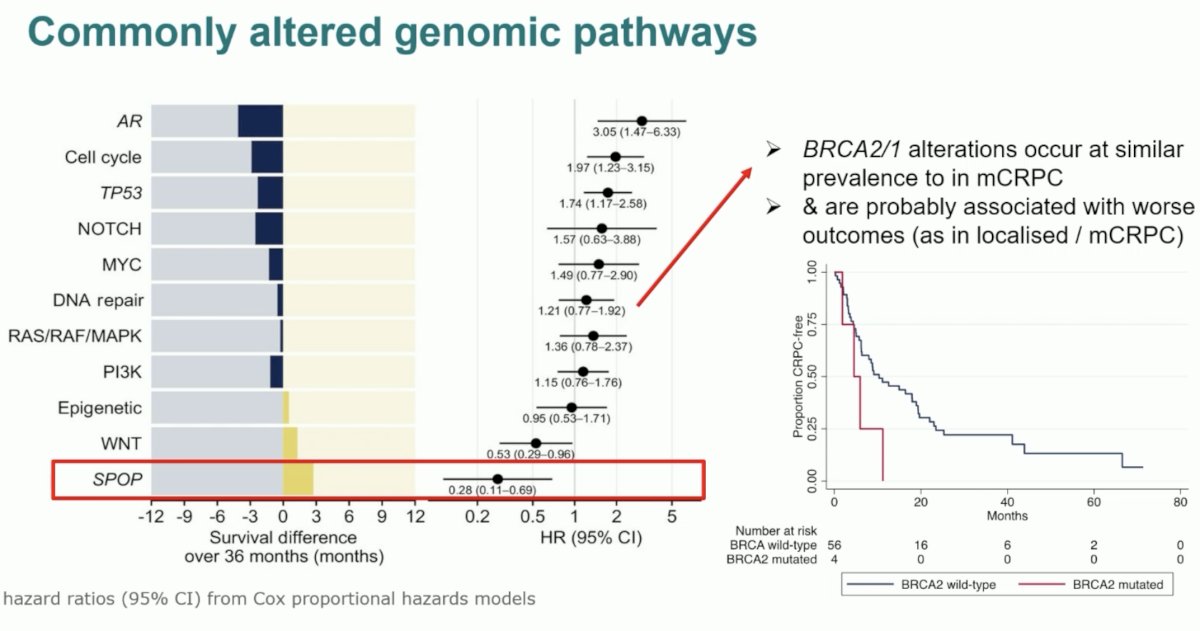
Dr. Attard noted that most genomic alterations in the mCRPC state appear to be present in the mCSPC state as well, with the main exception being androgen receptor pathway mutations. Another exception is likely the SPOP mutations, which are associated with favorable survival outcomes. Thus, these patients could be candidates for treatment de-escalation. However, conversely, BRCA1/2 mutations appear to occur at a similar frequency compared to the mCRPC state and are likely associated with worse outcomes across the disease spectrum.
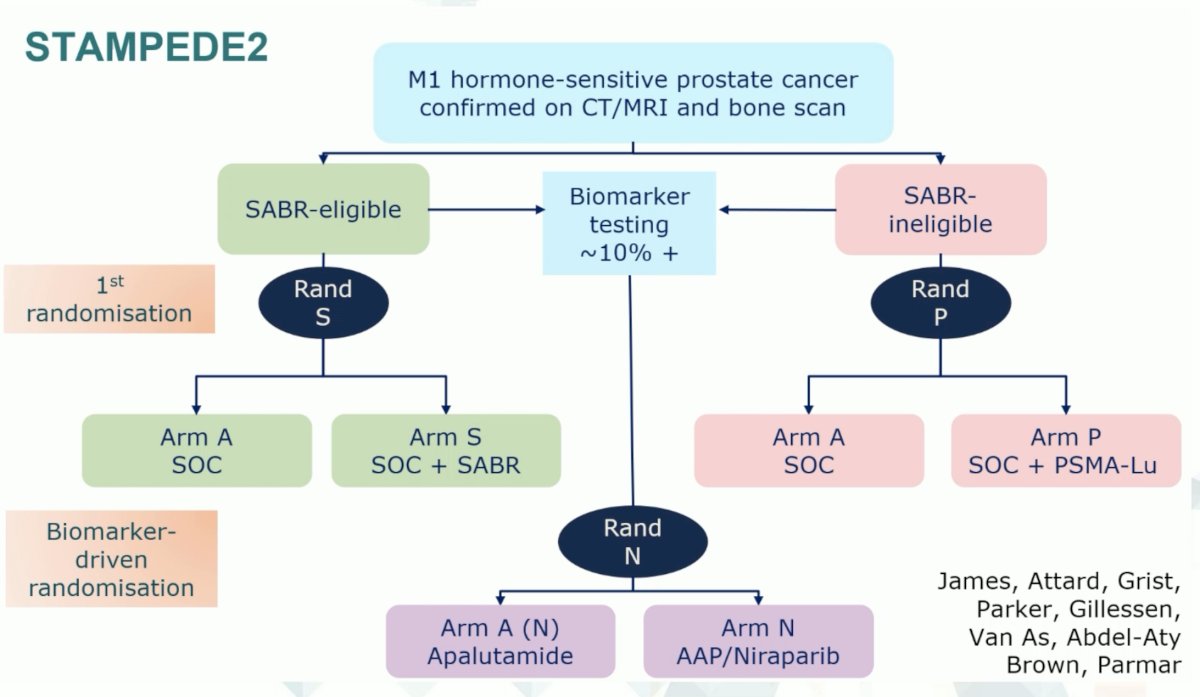
The STAMPEDE-2 multi-arm, multi-trial platform will address many of these questions. Patients with conventional-imaging defined, mCSPC will be randomized to evaluate the role of stereotactic body radiotherapy, Lu-PSMA, PARP inhibitors in biomarker positive patients, and treatment intensification with apalutamide, as summarized below:
Dr. Attard concluded his presentation as follows:
- ADT + an ARPI should be the backbone of treatment for all M1 and high-risk M0 advanced prostate cancer patients
- Molecular transcriptome expression-based tests could play a role in patient management, but additional datasets rare required for practice change
- Clinical implications of some biological processes appear to differ by metastatic state (tumour load at presentation/underling biology/different treatment paradigms)
- Artificial intelligence-derived models using digitized H&E images contain prognostic information in metastatic patients
- Biomarker-selective trials evaluating AKTi or PARPi + ADT + ARPIs are underway
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24(5):443-456.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol 2019 Dec 1;30(12):1992-2003.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomized controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022 Jan 29;399(10323):447-460.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials. Lancet Oncol. 2023;24(7):783-797.


