(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Andre Fay discussing patient reported outcomes in the phase 3 TALAPRO-2 study. TALAPRO-2 showed statistically significant improvement in imaging-based progression-free survival with talazoparib + enzalutamide (n = 200) vs placebo + enzalutamide (n = 199) (HR 0.45, 95% CI 0.33–0.61) in men with HRR mutations receiving first-line treatment for mCRPC.1 However, the impact of treatment on patient health related quality of life is important to consider along with potential clinical benefits, as maintaining or improving quality of life is a major goal of treatment for mCRPC. At the 2023 ESMO annual congress, Dr. Fay and colleagues reported patient-reported outcomes data for this HRR mutation cohort.
Patient-reported outcomes were assessed on day 1 and scheduled visits (every 4 weeks until week 53, then every 8 weeks) until radiographic progression using the EORTC QLQ-C30 and its prostate cancer module, QLQ-PR25. Prespecified patient-reported outcomes endpoints included overall mean change from baseline (per longitudinal repeated measures mixed-effects model) and time to definitive deterioration with a clinically meaningful change of ≥ 10 points. Stratified log-rank test and Cox proportional hazards model were used to make time to definitive deterioration between-arm comparisons.
Of the 399 men in the HRR mutation cohort, 394 (n = 197 both arms) had at least a baseline plus 1 post-baseline patient-reported outcomes score. The baseline demographics are as follows:

A longer time to definitive deterioration for talazoparib + enzalutamide vs placebo + enzalutamide was observed in global health status/quality of life (HR 0.69, 95% CI 0.49–0.97; median 27.1 vs 19.3 months):

Additionally, time to definitive deterioration in physical, emotional, and cognitive functioning, as well as nausea and vomiting, pain, appetite loss, and constipation favored talazoparib + enzalutamide vs placebo + enzalutamide. Statistical significant differences in mean scores over time for physical, emotional, and cognitive function and pain favored talazoparib + enzalutamide vs placebo + enzalutamide, however, none of these treatment differences observed met the pre-defined clinically meaningful threshold of >= 10 points. With regards to EORTC QLQ-PR25, time to definitive deterioration in urinary symptoms was significantly longer with talazoparib + enzalutamide vs placebo + enzalutamide (HR 0.56; 95% CI 0.34–0.93; p = 0.022; median not reached vs 30.2 months):
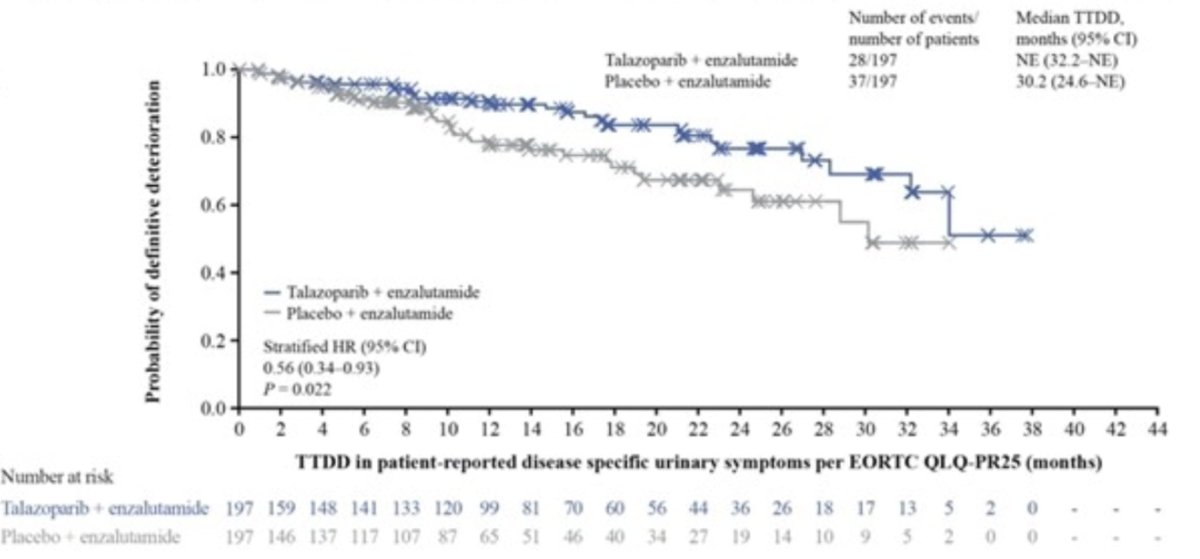
Dr. Fay concluded his presentation by discussing patient reported outcomes in the phase 3 TALAPRO-2 study with the following concluding statements:
- Time to definitive deterioration in global health status/quality of life and urinary symptoms were significantly longer with talazoparib + enzalutamide vs placebo + enzalutamide
- Modest treatment differences favoring talazoparib + enzalutamide vs placebo + enzalutamide was observed for some functioning scales
- These data complement the benefit–risk assessment of TALAPRO-2 for men with mCRPC who have HRR mutations
Presented by: Andre P. Fay, MD, PhD, PUCRS School of Medicine, Porto Alegre, Brazil
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:


