(UroToday.com) The 2024 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Dingwei Ye discussing BL-B01D1, an EGFR x HER3 bispecific antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma.
EGFR and HER3 are highly expressed in urothelial carcinoma, thus targeting EGFR and HER3 could provide a promising therapeutic option for urothelial carcinoma. BL-B01D1 is a potentially first-in-class antibody-drug conjugate comprised of an EGFR x HER3 bispecific antibody attached to a novel topoisomerase I inhibitor payload (Ed-04) via a tetrapeptide-based cleavable linker: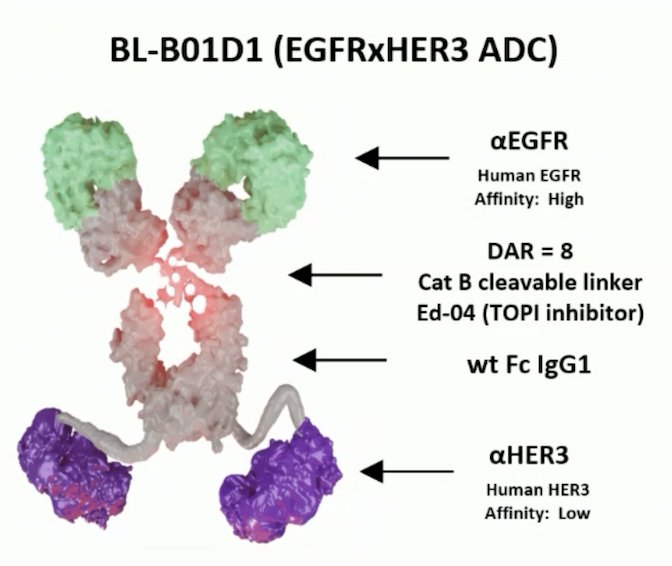
At ESMO 2024, Dr. Ye and colleagues presented safety and efficacy data from a phase Ib/II study of BL-B01D1 in urothelial carcinoma.
This phase Ib/II study included patients with locally advanced or metastatic urological tumors that had failed standard therapy or without feasible treatment options. Patients had to have measurable disease per RECIST v1.1 and have an ECOG performance status of 0-1. The enrolled urothelial carcinoma patients were mainly administered at doses of 2.2, 2.5, and 2.75 mg/kg D1D8 every 3 weeks. The primary endpoint of this trial was objective response rate by investigator’s assessment and the trial design for BL-B01D1-201 is as follows: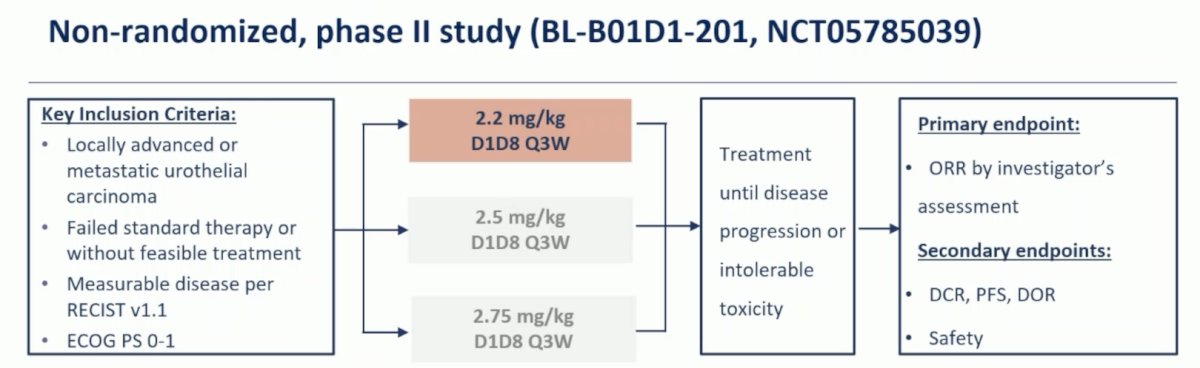
As of June 30, 2024, 41 urothelial carcinoma patients were enrolled in an every 3-week treatment schedule, with 34 patients treated at 2.2 mg/kg, 4 patients at 2.5 mg/kg, and 3 patients at 2.75 mg/kg. The median prior line of systemic treatment was 2 (range: 1-7). The baseline characteristics stratified by dose are as follows: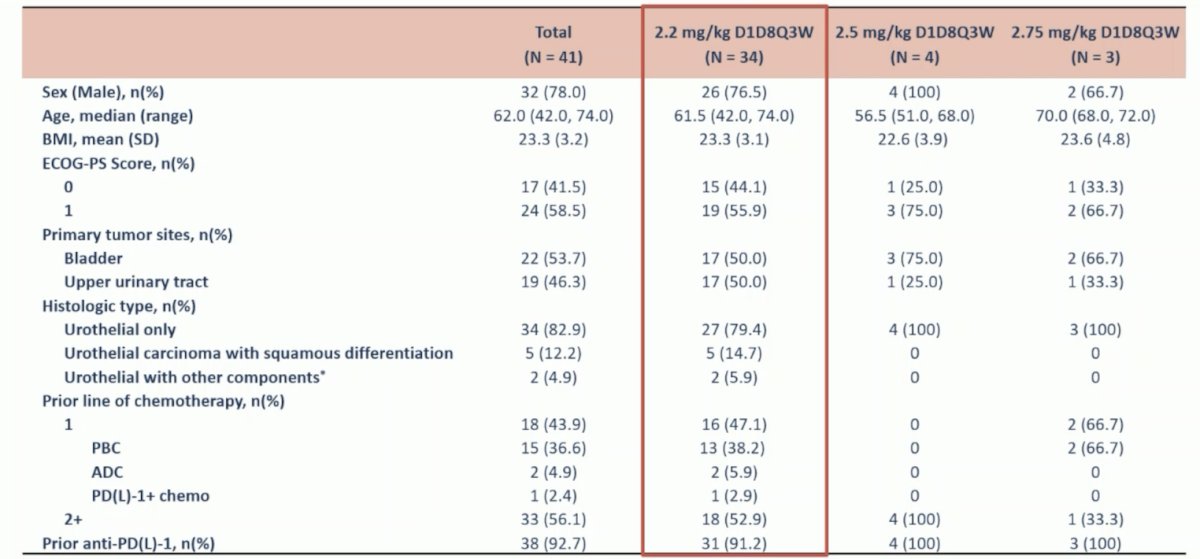
Among the enrolled patients, 27 patients dosed at 2.2 mg/kg were evaluated for efficacy, objective response rate was 40.7% (95% CI 22.4-61.2), clinical objective response rate was 33.3% (95% CI 16.5-61.2), disease control rate was 96.3% (95% CI 81.0-99.9), and median progression-free survival was not reached (95% CI 4.2-not reached). For patients pretreated with one chemotherapy line (n = 12), the objective response rate was 75.0% (95% 42.8-94.5), the clinical objective response rate was 75.0% (95% CI 42.8-94.5), and the median progression free survival was not reached (95% not reached – not reached):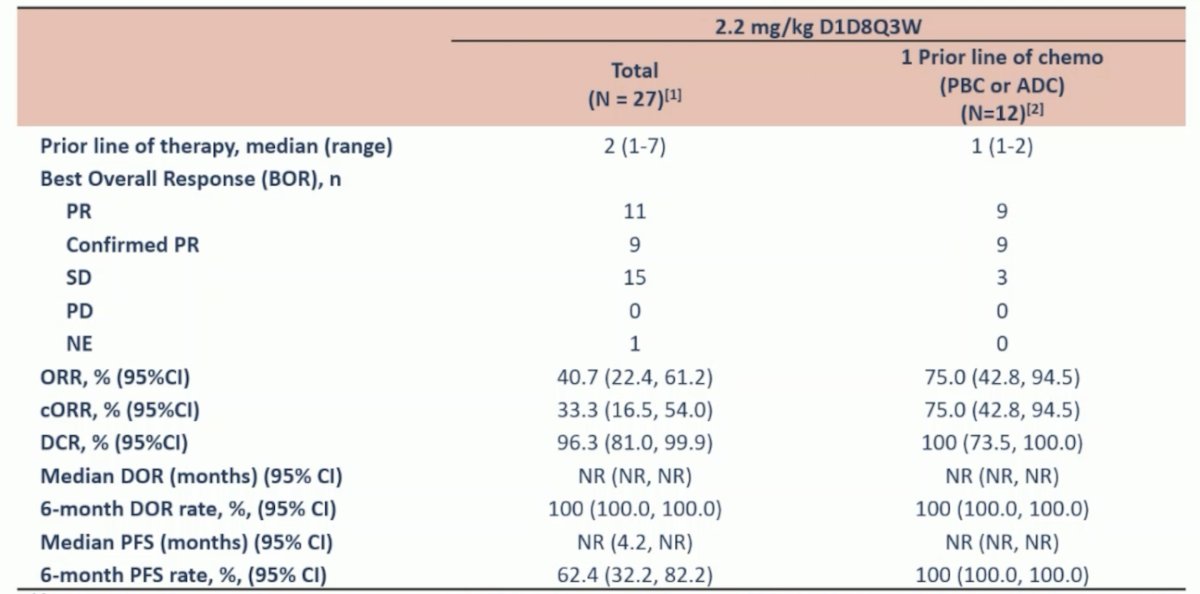
For patients treated at the 2.2 mg/kg dose, the 6-month duration of response rate was 100%: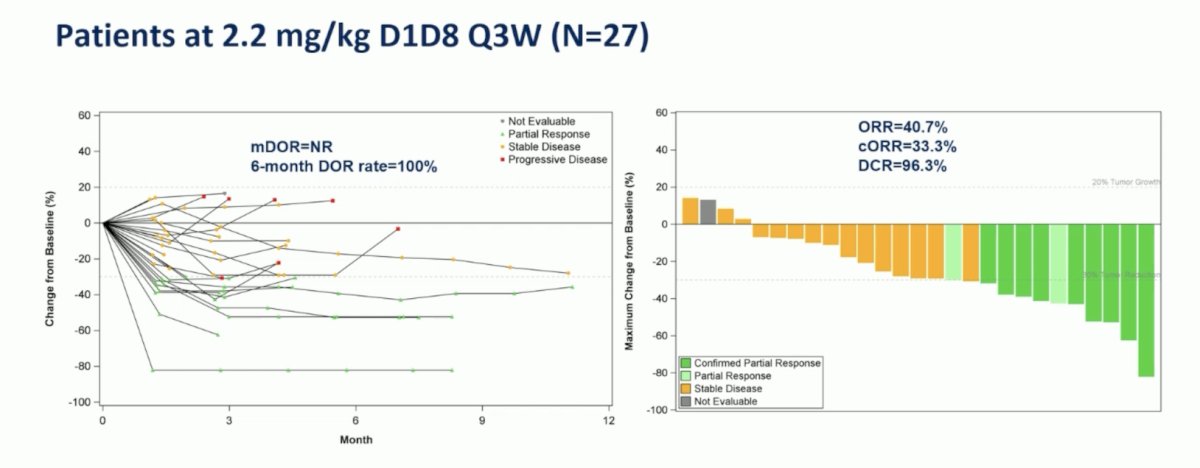
For patients treated at the 2.2 mg/kg dose with one prior line of chemotherapy, the 6-month duration of response rate was also 100%: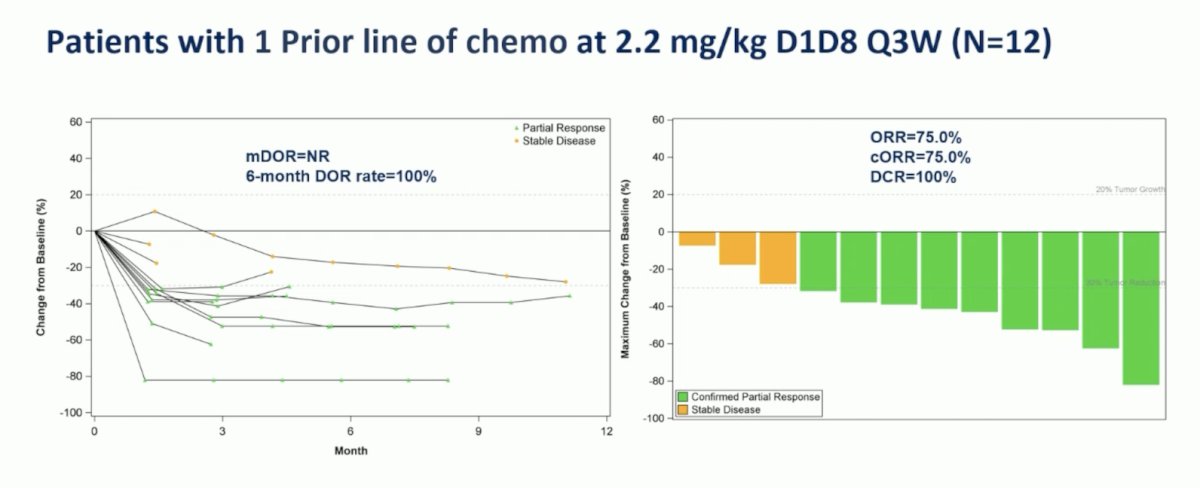
Dr. Ye also noted that clinical activity was seen across various levels of EGFR and HER3 expression: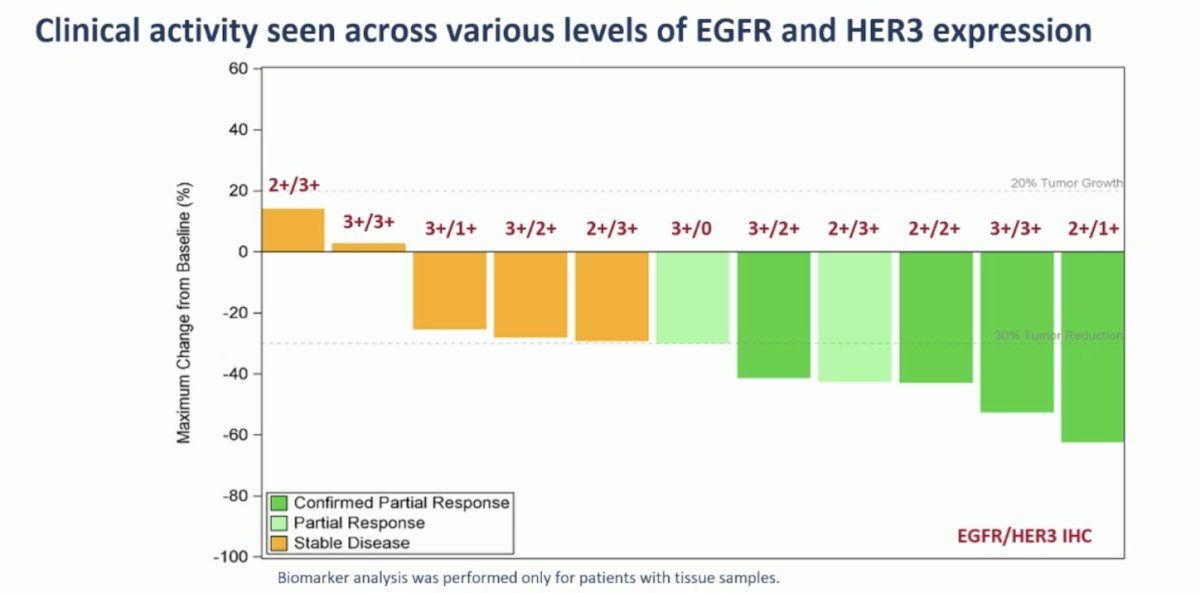
Over a median follow-up of 4.6 months, all patients had a treatment-related adverse event, but none that led to death, 5.9% of which led to discontinuation, and 14.7% of which led to dose reduction. Treatment-related adverse events occurring in >= 15% of patients at the 2.2 mg/kg dose are highlighted below: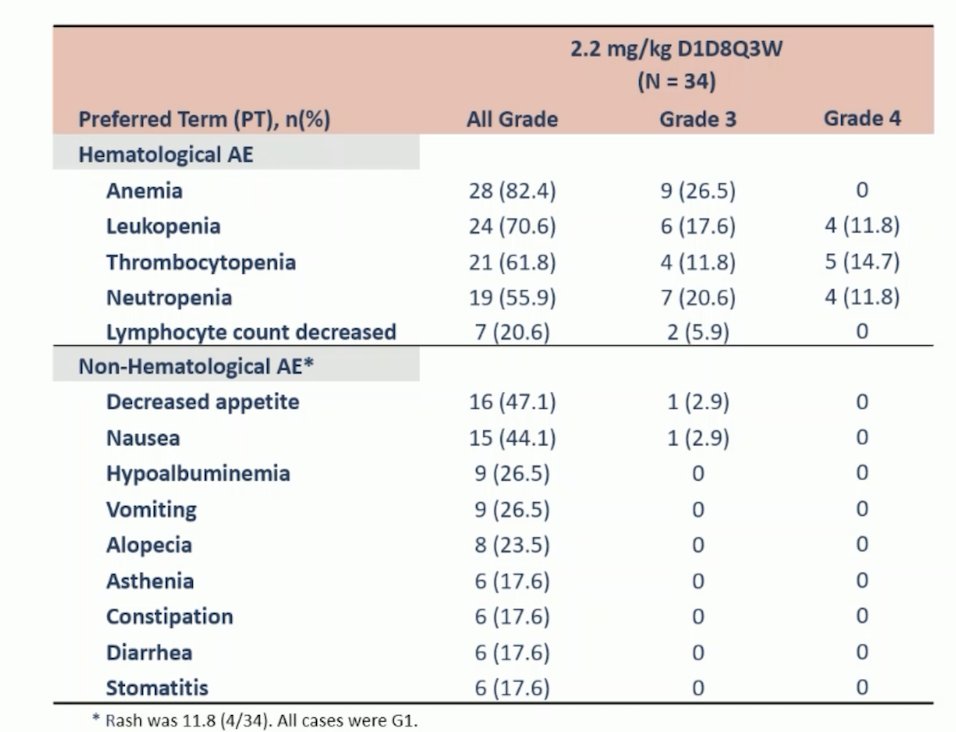
No interstitial lung disease was observed, and no new safety signals were observed.
Dr. Ye concluded his presentation discussing BL-B01D1 in patients with locally advanced or metastatic urothelial carcinoma with the following take-home points:
- BL-B01D1 showed encouraging preliminary efficacy and favorable safety at 2.2 mg/kg D1D8 Q3W in previously treated urothelial carcinoma, especially in the second-line setting
- Biomarker analysis demonstrated that clinical activity was seen across various levels of EGFR and HER3 expression
- The most common treatment-related adverse events were hematological toxicities, which were manageable
- The incidence and severity of toxicities related to EGFR and HER3 targeting were relatively low, and no new safety signals were observed.
- Given the promising results, plans are underway for registrational trials
Presented by: Dingwei Ye, Professor, Fudan University Shanghai Cancer Center, Shanghai, China
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: New Dual-Target Drug Shows Promise Against Bladder Cancer - Jonathan Rosenberg


