(UroToday.com) The 2024 ESMO annual meeting included a session on expanding bladder preservation by optimal use of systemic therapy and biomarkers, featuring a presentation by Dr. Srikala Sridhar discussing the potential of optimal systemic therapy without locoregional therapy.
Dr. Sridhar notes that muscle-invasive bladder cancer is an aggressive disease, with the standard of care being neoadjuvant chemotherapy followed by radical cystectomy. However, pathological complete response rates are ~25%, but can only be accurately determined by a radical cystectomy. Some patients are ineligible for surgery, a growing number are unfit, and some eligible patients decline radical cystectomy. So, several questions remain:
- How do we optimize our bladder-sparing strategies?
- Can we preserve more bladders that have had a pathological complete response?
- Can we get to a point where the default is not radical cystectomy for all patients?
Dr. Sridhar notes that there are several key points of consideration for bladder-sparing strategies:
- Careful patient selection: staging, biomarkers
- Use of more effective systemic therapy regimens
- Improving the accuracy of response assessment
- Detecting and treating local recurrences early to prevent metastatic disease
There are two scenarios we need to avoid: (i) overtreatment, using multiple modalities if a cure can be achieved with just one modality, and (ii) undertreatment, missing an opportunity to cure because of treatment de-escalation. One of the major historical barriers to a bladder-sparing approach has been limited prospective studies, and a lack of harmonized approaches, including patient selection/biomarkers, limited use of neoadjuvant therapies, and active surveillance or trimodality therapy. Moreover, there is a lack of standardized endpoints in bladder-sparing trials:
- No pathological complete response, so we need to validate the clinical complete response
- Disease-free survival, overall survival, and what time point should we evaluate bladder-intact disease-free survival
Dr. Sridhar emphasized that perhaps we can achieve bladder sparing with systemic therapy alone using a risk-adapted approach:
Early studies of TURBT + chemotherapy from major cancer centers showed favorable outcomes with a bladder-sparing approach.1 In a study of 148 patients from Memorial Sloan Kettering Cancer Center and Columbia University that had a clinical complete response to neoadjuvant chemotherapy and elected for active surveillance, the 5-year disease-specific, overall, cystectomy-free and recurrence-free survival rates were 90%, 86%, 76%, and 64%, respectively. Dr. Sridhar suggests that in carefully selected and closely monitored patients, this approach could be considered, but these data also highlight the need to identify biomarkers and novel imaging approaches for patient selection.
The RETAIN study looked at risk-enabled therapy after neoadjuvant chemotherapy and included patients with cT2-3N0M0 bladder cancer who received accelerated MVAC followed by a second, re-staging TURBT. The treatment approach was risk-adapted based on residual disease and mutational statuses: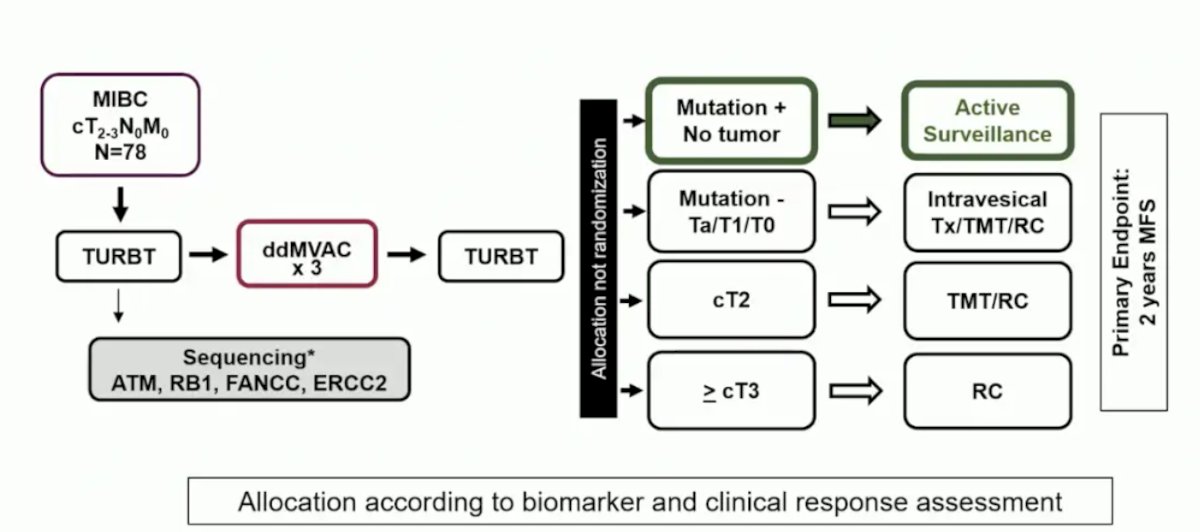
In the ITT population, 33 (46%) patients had a relevant mutation and 26 (37%) began active surveillance. The full treatment of the patients with mutation-positive disease is summarized below: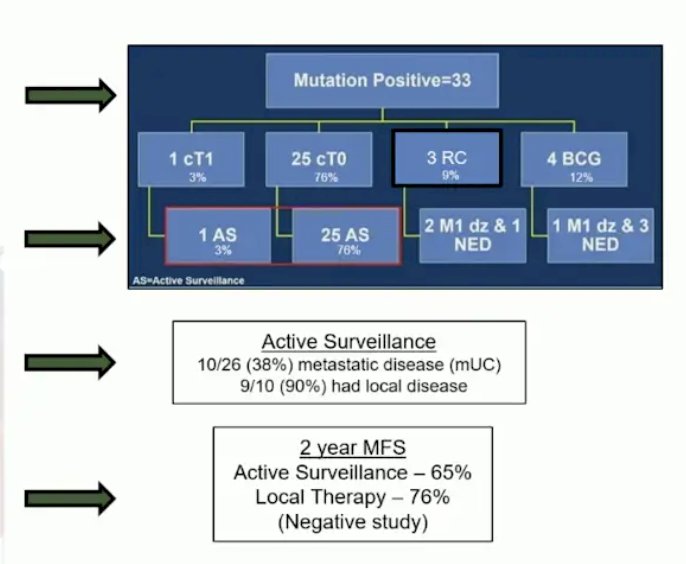
Dr. Sridhar noted the following questions that arise from the RETAIN study:
- Why did patients who were mutation-positive and on active surveillance develop metastatic disease?
- Can clinical complete response assessment be enhanced with additional imaging or ctDNA?
- Are we undertreating these patients? Would the use of trimodality therapy reduce the risk of local recurrence or metastases?
- Should immunotherapy be incorporated into this bladder-sparking approach?
Based on these aforementioned concerns, RETAIN 2 is incorporating immunotherapy, with the following trial design: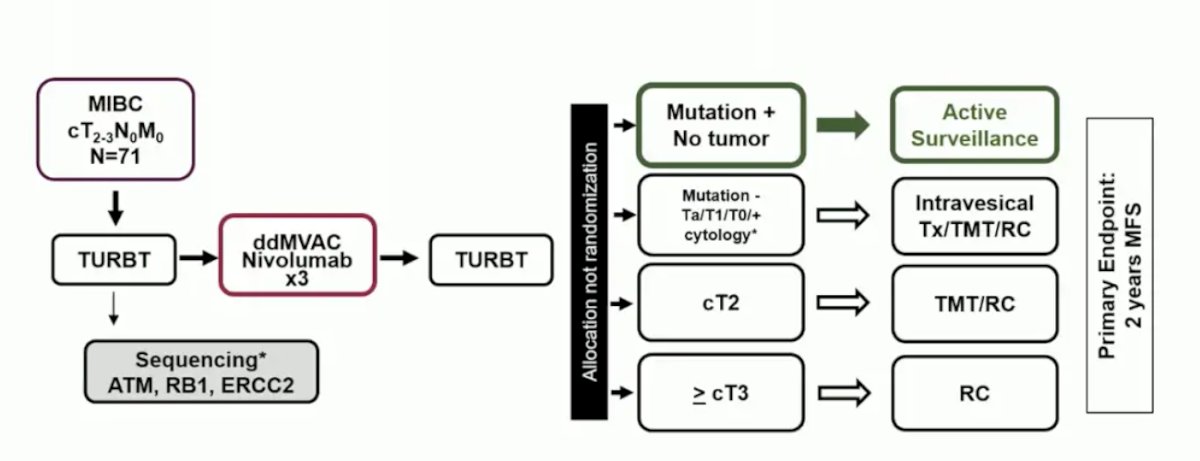
Another ongoing trial incorporating a risk-adapted approach is the ALLIANCE A031701 trial: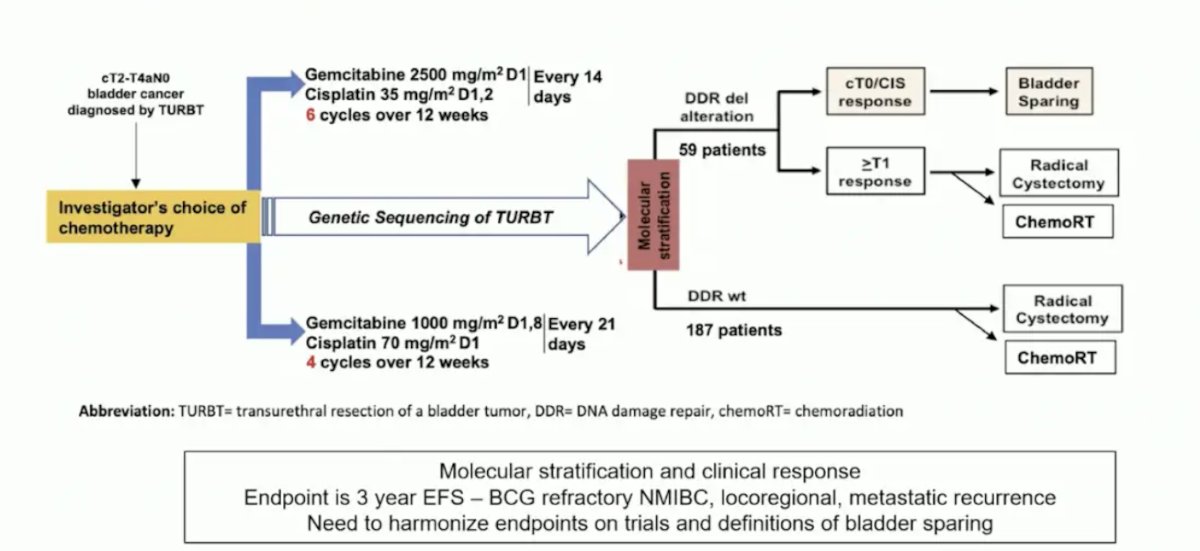
An important question is whether we can move first-line metastatic urothelial carcinoma treatments to the muscle-invasive bladder cancer setting. In HCRN GU 16-257, patients received nivolumab + gemcitabine/cisplatin chemotherapy x 4 cycles, after which they were re-staged with cystoscopy + biopsy, urine cytology, and MRI. Those with evidence of a complete clinical response were recommended to forgo cystectomy and received adjuvant nivolumab every 2 weeks for a total of 8 cycles. The primary endpoint was 2-year metastasis-free survival or <ypT1N0 in the patients with a complete clinical response: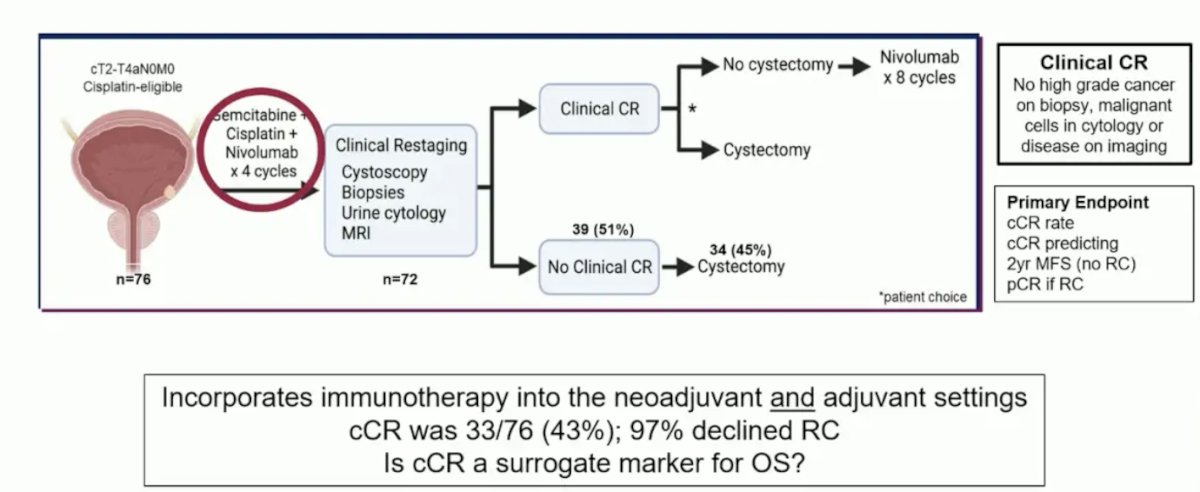
Among the 72 patients who were recruited and subsequently underwent clinical re-staging, 33 patients (46%) had evidence of a complete clinical response. One patient opted for immediate cystectomy, with the remaining 32 proceeding with active surveillance. Overall, 1 patient developed metastases each before and after 2 years follow-up. Of these 2, 1 had died of their metastatic disease, and 9 patients underwent a cystectomy during a minimum follow-up of 19 months: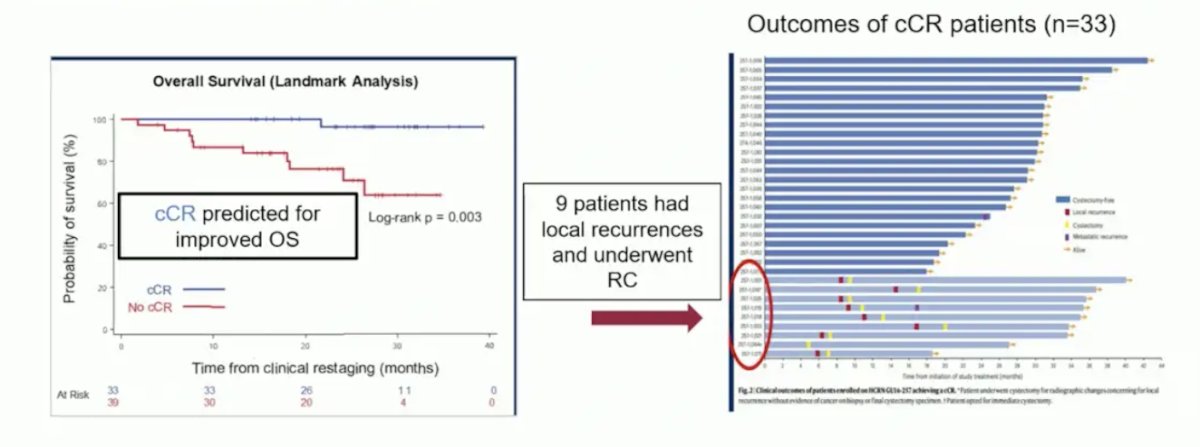
An important question is whether trimodality therapy would have reduced or prevented some of these local recurrences and the need for radical cystectomy. There are several ongoing perioperative trials with enfortumab vedotin combinations, including EV303, VOLGA, and EV304: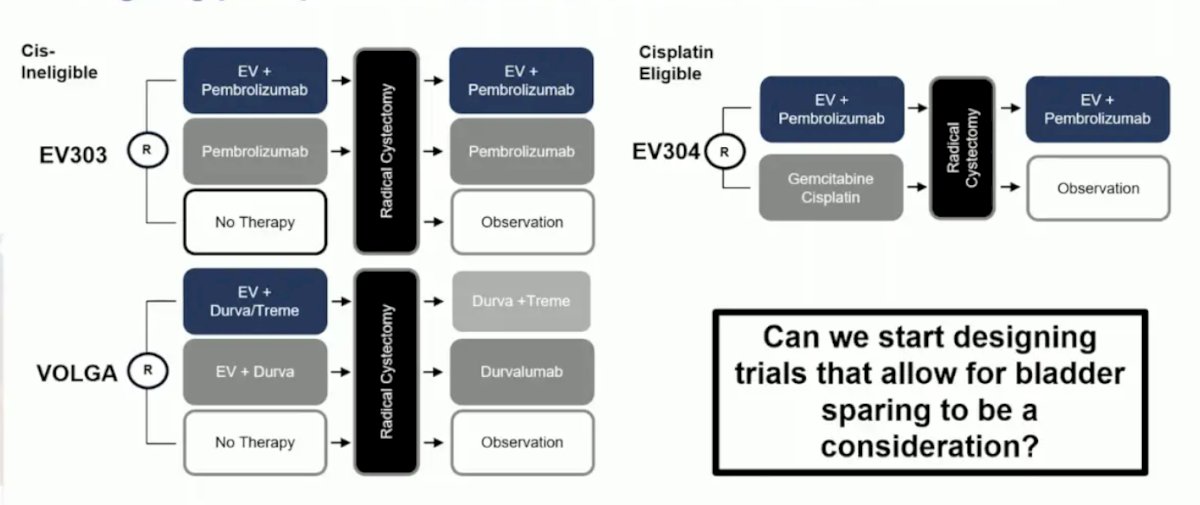
Dr. Sridhar noted that in the SURE 01 trial of neoadjuvant sacituzumab, six patients had a complete response (cystoscopy, MRI, and ctDNA), and subsequently declined radical cystectomy. This brings up the point that as neoadjuvant treatment gets better, and more clinical complete responses are seen, trials need to be designed to account for patient preference. The following highlights bladder-sparing trial design considerations, taking into account patient considerations, systemic treatment, local treatment, and endpoints: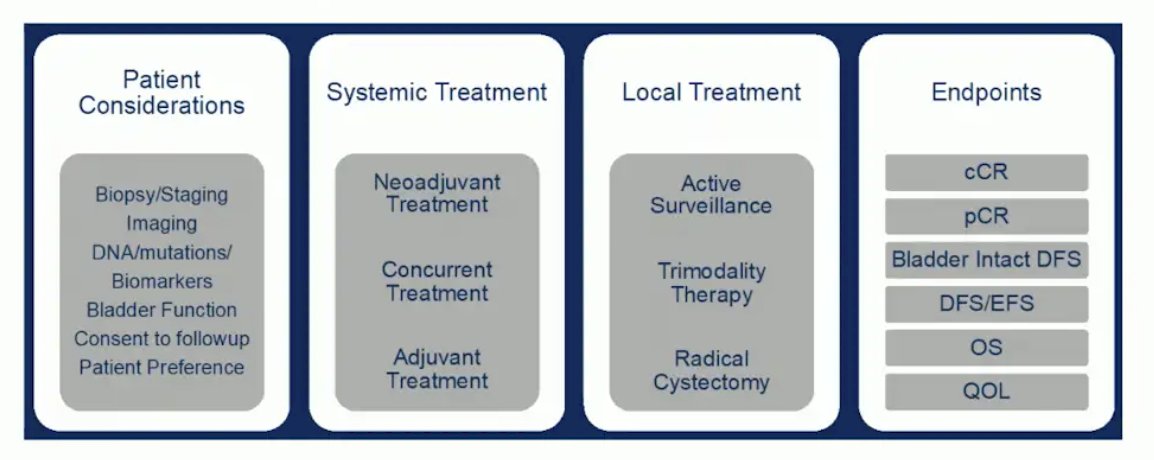
Dr. Sridhar concluded her presentation discussing the potential of optimal systemic therapy without locoregional therapy with the following take-home points:
- As more active systemic combinations enter the muscle-invasive bladder cancer disease space, the question will be whether this will suffice or some form of local therapy is still needed
- Trimodality therapy still allows patients to preserve their bladder and is well-tolerated
- It will be critical to validate relevant biomarkers to help in patient selection
- Harmonizing endpoints and establishing clear criteria for establishing clinical complete response will be key
- If more patients can keep their bladders, especially those bladders that are functioning well, this may lead to improved quality of life.
- To maximize outcomes, we need a highly collaborative multidisciplinary approach.
Presented by: Srikala S. Sridhar, MD, MSc, FRCPC, Professor, Princess Margaret Cancer Centre, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Mazza P, Moran GW, Li G, et al. Conservative management following complete clinical response to neoadjuvant chemotherapy of muscle-invasive bladder cancer: Contemporary outcomes of a multi-institutional cohort study. J Urol. 2018 Nov;200(5):1005-1013.


