(UroToday.com) The 2024 ESMO annual meeting included a session on expanding bladder preservation by optimal use of systemic therapy and biomarkers, featuring a presentation by Dr. Eva Schaake discussing biomarkers and prediction of clinical complete response. The prognosis of stage II-III urothelial cancer is poor, with a 5-year overall survival of only 25-47% after radical cystectomy. Cisplatin-based neoadjuvant chemotherapy can modestly improve overall survival, but there is no biomarker for response in clinical practice. Immunotherapy combination trials show encouraging activity and may have less cross resistance with chemo-radiotherapy. Currently, there are better bladder sparing options emerging with increasing efficacy of new treatment combinations and improved trimodality treatment. As such, biomarkers could help select candidates for bladder-sparing options, especially in high-risk disease.
Dr. Schaake asks: Can we predict response to neoadjuvant/induction chemotherapy? To date, genomic biomarkers for chemotherapy have included DNA damage response genes. For example, the presence of one or four gene mutations (ATM, RB1, FANCC, ERCC2) has been shown to improve response to neoadjuvant cisplatin-based chemotherapy. However, the predictive nature of these genomic alterations has not been confirmed in additional studies. ERCC2 mutations have been shown to predict response in several studies, however mutation frequency is low (10% at most). Thus, ERCC2 is not yet ready for clinical application.
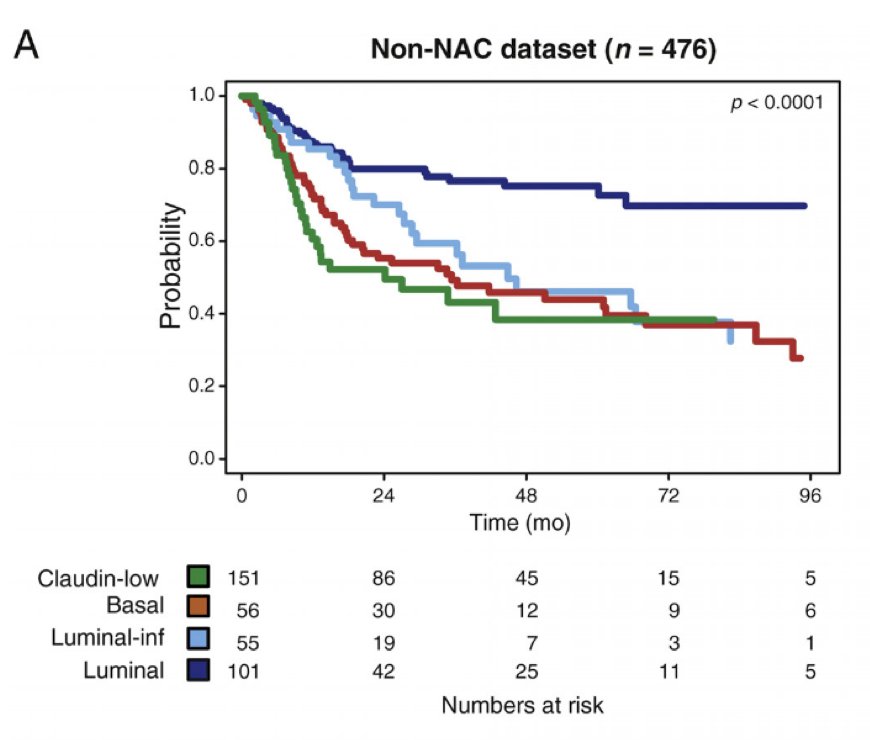
Arguably the most clinically relevant predictor for response to chemotherapy is molecular subtyping. Dr. Schaake notes that the luminal molecular subtype appears to have less aggressive clinical presentation (in many cases), reflected by good outcomes, with data indicating that luminal tumors are more likely to be organ-confined (pT1-2N0) at surgery. Non-organ confined tumors have greater benefit from neoadjuvant chemotherapy. Seiler et al.1 assessed pre-neoadjuvant chemotherapy transurethral specimens from 343 patients with muscle-invasive bladder cancer. Overall survival according to subtype was analyzed and compared with OS in 476 non-neoadjuvant chemotherapy cases (published datasets). Luminal tumors had the best overall survival with and without neoadjuvant chemotherapy:
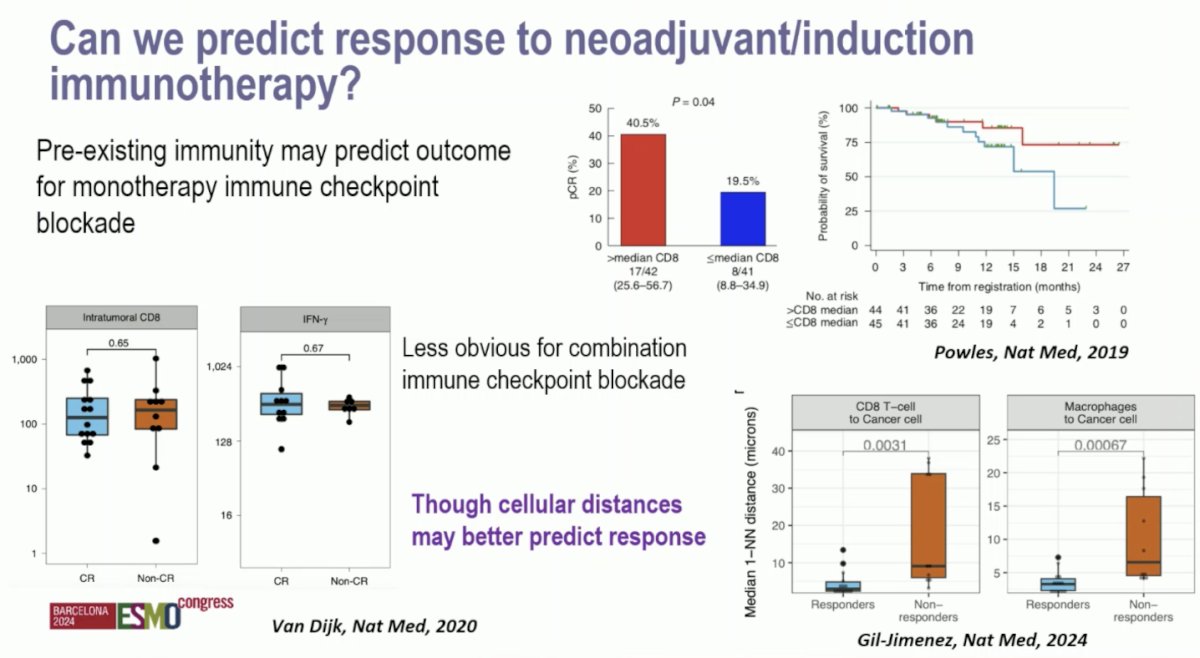
Can we predict response to neoadjuvant/induction immunotherapy? Pre-existing immunity may predict the outcome of monotherapy immune checkpoint blockade. However, this is less obvious for combination immune checkpoint blockade. Moreover, cellular distances may better predict response:
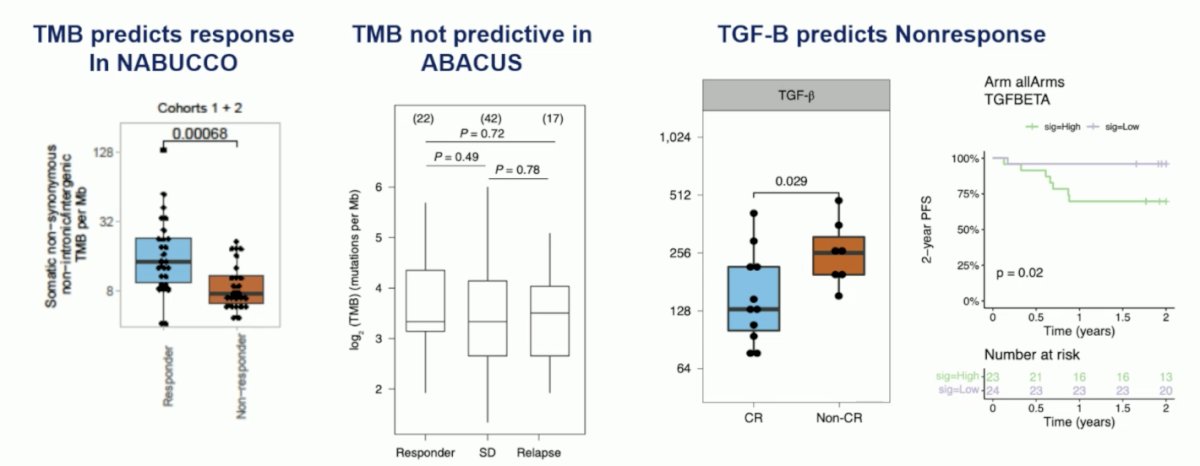
Other biomarkers may predict response to preoperative immunotherapy. Tumor mutational burden predicted response in NABUCCO, although it was not predictive in ABACUS. TGF-Beta has also been shown to predict non-response:
Can we predict enfortumab vedotin + IO response to therapy? Membranous NECTIN4 expression correlates with enfortumab vedotin response and outcomes. Additionally, NECTIN4 copy number alterations occur in approximately 25% of metastatic urothelial carcinoma cases, which is associated with strong membranous NECTIN4 expression. Patients with NECTIN4 amplification exhibit an objective response rate of >90% to enfortumab vedotin monotherapy and is associated with long term survival. But, do we need predictive biomarkers for enfortumab vedotin + pembrolizumab? Based on the EV-302 trial,2 there is clearly a high response rate in stage IV disease with the combination therapy, with even higher responses in lymph node only advanced urothelial carcinoma. Thus, is it necessary to have a biomarker if excellent response is replicated in the neoadjuvant/induction setting?
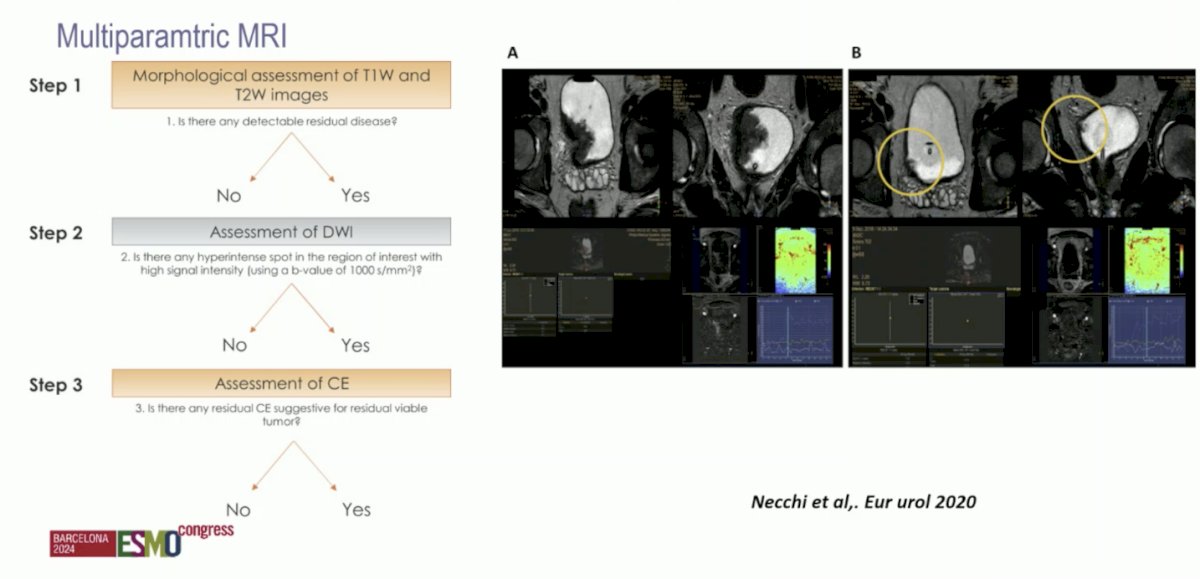
With regards to imaging, can we predict response to therapy with multiparametric MRI (mpMRI)? Andrea Necchi and colleagues previously showed that mpMRI may be a noninvasive modality for assessing tumor response to neoadjuvant pembrolizumab in muscle-invasive bladder cancer based on data from the PURE-01 trial:3
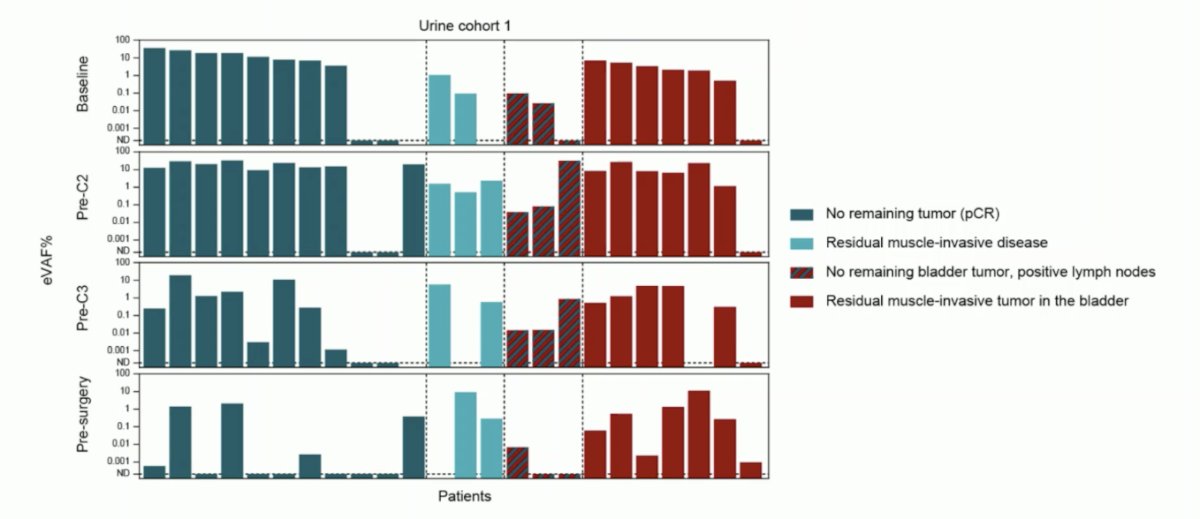
Can we predict response on therapy with ctDNA? ctDNA has previously been shown to be a prognostic marker for systemic tumor load/presence, and plasma ctDNA has been shown to be able to track tumor response in neoadjuvant chemotherapy or immune checkpoint blockade. Further, an absence of ctDNA post-cystectomy indicates patients with good outcomes, and presence of ctDNA indicates better outcomes with atezolizumab versus observation. A recent systematic review by Crupi et al.4 assessed the evidence of ctDNA as a prognostic and predictive biomarker in the perioperative treatment of muscle invasive bladder cancer. Among 6 studies included, the investigators confirmed the prognostic role of ctDNA after cystectomy and shows a potential predictive benefit in using neoadjuvant chemotherapy and preoperative immunotherapy. Circulating tumor DNA was used to monitor recurrence, and changes in ctDNA status anticipated radiological progression with a median difference of time from 101 to 932 days. van Dorp et al.5 looked at the impact of urine ctDNA in the NABUCCO trial, finding that the absence of urinary ctDNA correlated with pathologic complete response in the bladder (ypT0Nx), but not with progression-free survival. However, absence of plasma ctDNA correlated with both pathologic complete response (OR 45.0; 95% CI 4.9-416.5) and progression free survival (HR 10.4, 95% CI 2.9-37.5):
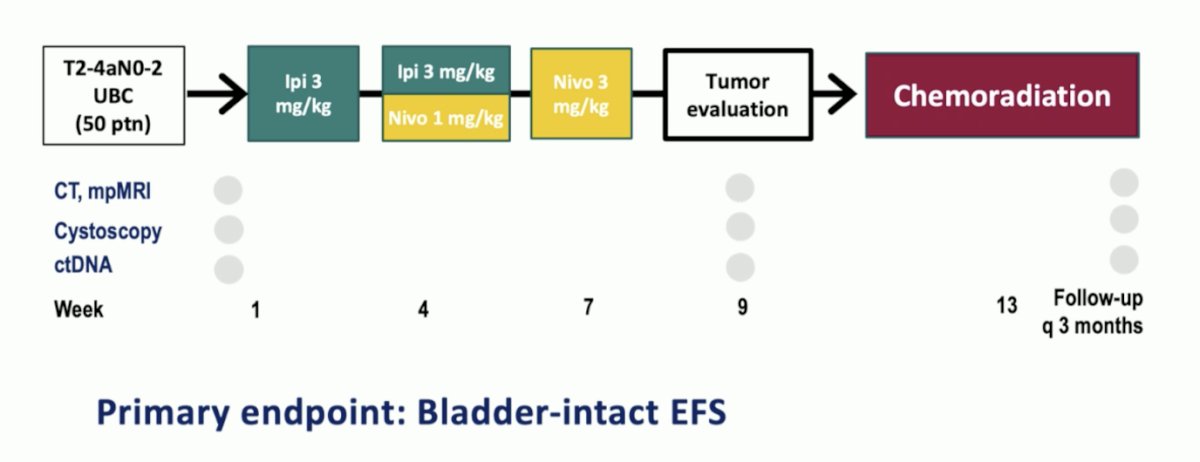
Finally, Dr. Schaake highlighted the INDIBLADE trial in progress assessing pre-treatment ipilimumab + nivolumab prior to chemoradiation and the utilization of ctDNA during treatment assessment:
Dr. Schaake concluded her presentation by discussing biomarkers and prediction of clinical complete response with the following take-home points:
- There have been significant advances in bladder cancer treatment, moving towards more organ preservation
- To date, molecular subtypes are still unreliable in clinical use
- Plasma ctDNA has shown response prediction on the systemic level
- For local status, we should be considering urine ctDNA, mpMRI, and cystoscopy
Presented by: Eva Schaake, Netherlands Cancer Institute, Amsterdam, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Seiler R, Al Deen Ashab H, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017 Oct;72(4):544-554.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.
- Necchi A, Bandini M, Calareso G, et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-invasive bladder cancer: Preliminary findings from the PURE-01 Study. Eur Urol. 2020 May;77(5):636-643.
- Crupi E, de Padua TC, Marandino L, et al. Circulating tumor DNA as a predictive and prognostic biomarker in the perioperative treatment of muscle-invasive bladder cancer: A Systematic review. Eur Urol Oncol. 2024 Feb;7(1):44-52.
- Van Dorp J, Pipinikas C, Suelmann BBM, et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat Med. 2023 Mar;29(3):588-592.


