(UroToday.com) The 2024 ESMO annual meeting included a session on addressing uncertainties in the management of urothelial and renal cell carcinomas, featuring a presentation by Dr. Bernadett E. Szabados discussing circulating versus tissue-based biomarkers.
Dr. Szabados prefaced her presentation by noting that biomarkers are a huge topic, and in the interest of time she would be providing a high-level overview of circulating and tissue-based biomarkers. For urothelial carcinoma there are established (FGFR3, PD-L1, ctDNA, and HER2) biomarkers and experimental (RNA-based molecular subtypes, tumor mutational burden, TGF-beta, Nectin-4, and Trop-2) biomarkers.
Dr. Szabados notes that FGFR3 expression is highest in Ta tumors, lower in T1 tumors, and even lower in T2 tumors. Erdafitinib has been used to target FGFR-positive metastatic urothelial carcinoma, with FGFR3 alterations including: G370C, R248C, S249C, Y373C, and FGFR2/3 fusions. These can be assessed using central laboratory testing using an RNA-based RT-PCR assay, or next-generation sequencing in a local laboratory (from either tissue or blood). Dr. Szabados notes that interestingly, despite high levels of FGFR expression in upper tract urothelial carcinoma, response to erdafitinib has been poor, with these tumors having better response with pembrolizumab: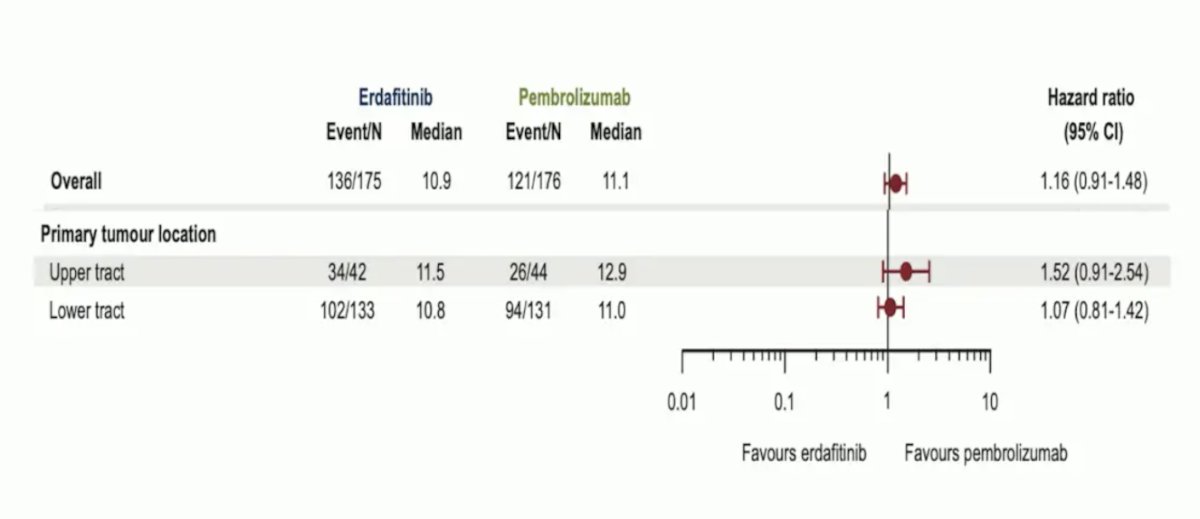
Trials such as the BISCAY trial, a biomarker-directed study in metastatic urothelial carcinoma, will continue providing precision treatment options for patients based on their tumor DNA analysis.
With regards to PD-L1, Dr. Szabados emphasized that this can be tested either via the immune cell or the tumor cell. Despite heterogeneity in outcomes over the last decade, generally, immune cell testing appears to be more prognostic, and tumor cell testing seems to be somewhat predictive in some trials: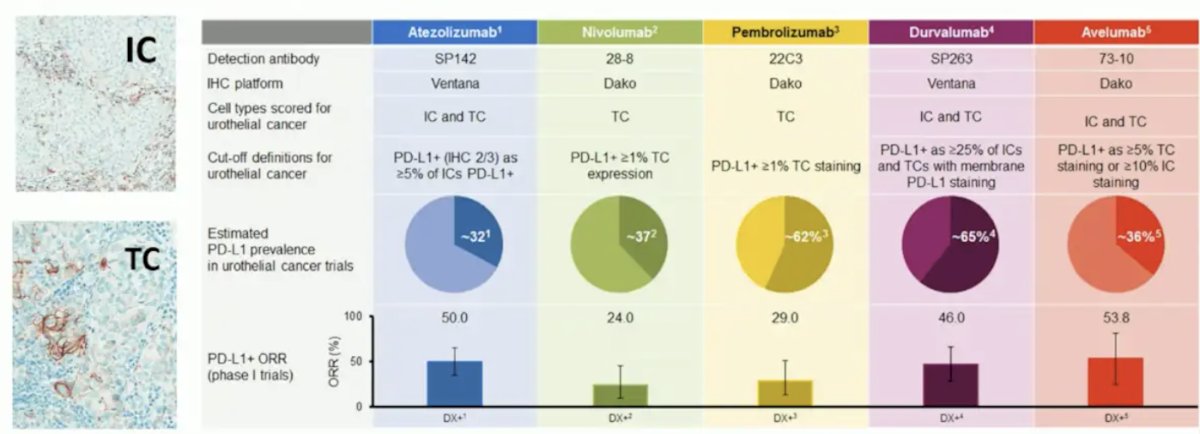
PD-L1 as a biomarker was initially promising for patient selection in the phase I PCD4989g study and phase II IMvigor210 cohort 2 trial: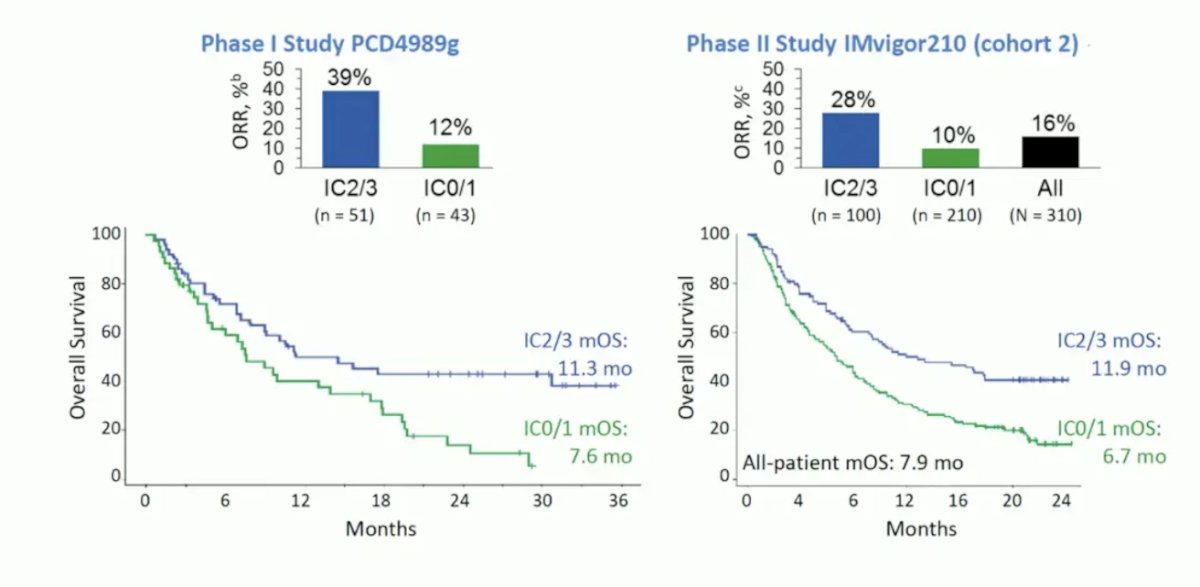
However, more recently, there have been more modest outcomes in PD-L1 positive patients when assessed in IMvigor211 and the first-line DANUBE trials: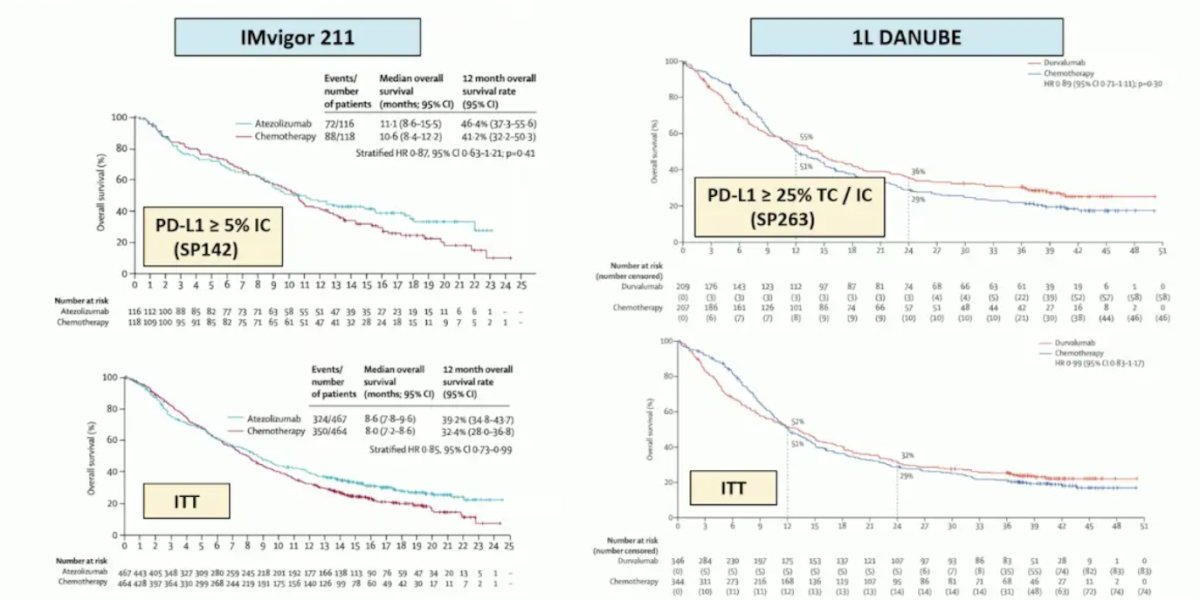
In the adjuvant setting, there has been similar heterogeneity, noting prognostication in the CheckMate 274 trial, but with very little value in the AMBASSADOR trial: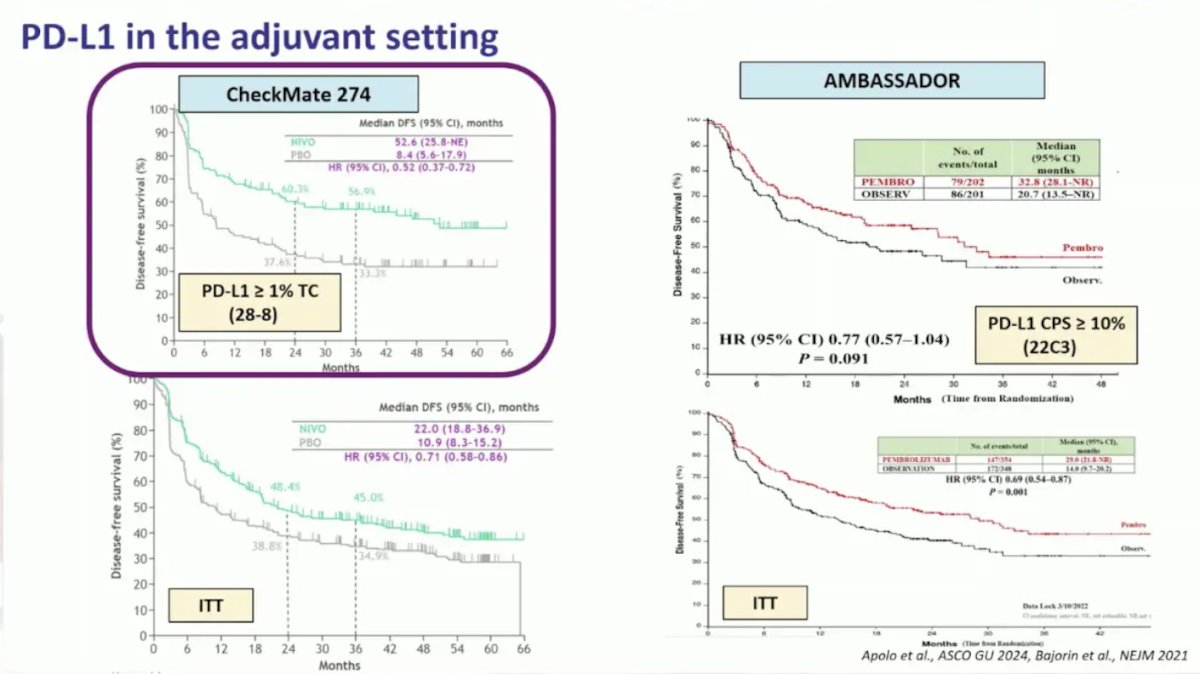
Next, Dr. Szabados addressed tumor mutational burden, which similar to PD-L1, showed promise ~8-10 years ago with TMB-high patients having longer survival with atezolizumab, but this was never prospectively tested. With regards to TGF-beta, high levels have been correlated with resistance to immunotherapy.
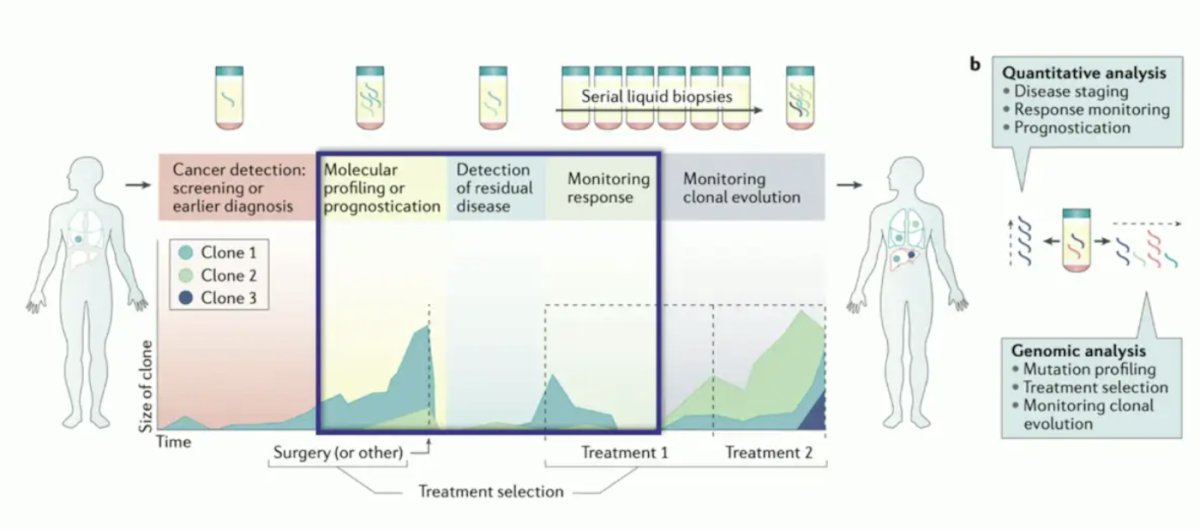
Dr. Szabados then spent some time discussing liquid biopsies and circulating tumor DNA (ctDNA). With ctDNA, we are measuring the DNA of circulating tumor cells, as well as proteins, extracellular vesicles, and host cells (immune cells, CAFs, and TAMs). ctDNA can then be used to assess gene panels and tumor-informed personalized assays. For urothelial carcinoma, previous work has shown that ctDNA correlates with disease burden (ctDNA levels increasing from localized to metastatic disease) and disease prognosis (patients with low ctDNA having improved survival):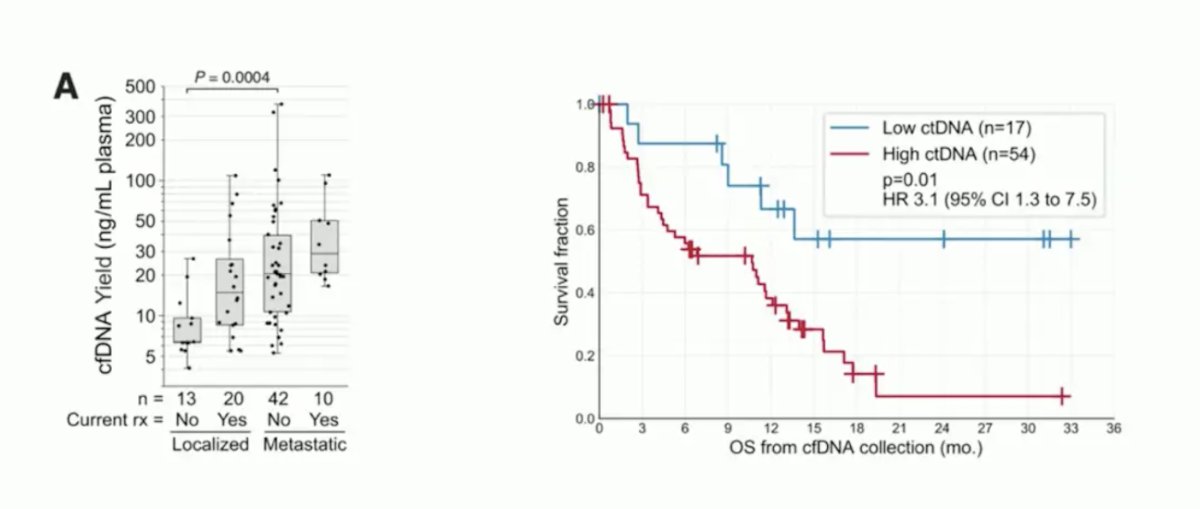
Current clinical ctDNA applications include:
- Cancer detection screening or earlier diagnosis
- Molecular profiling or prognostication
- Detection of residual disease
- Monitoring response to treatment
- Monitoring clonal evolution
From the IMvigor010 trial, Powles et al.1 showed that ctDNA is very prognostic in the adjuvant setting for muscle-invasive bladder cancer, particularly in the observation arm for assessing disease-free and overall survival: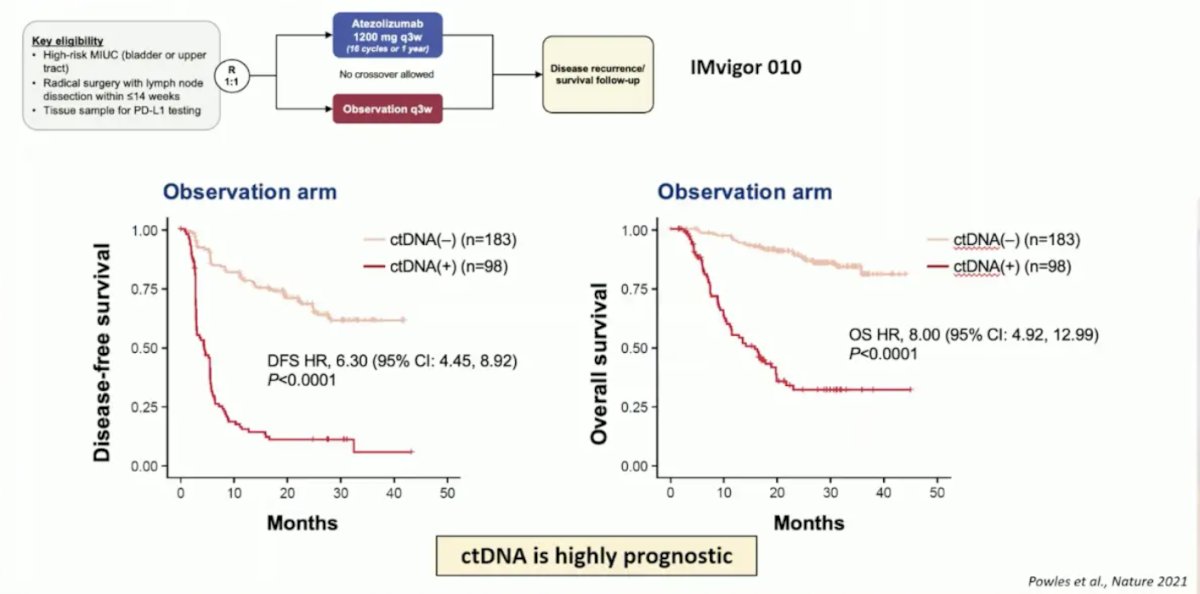
Additionally, in this same analysis, ctDNA was prognostic for assessing minimal residual disease and correlating with disease-free and overall survival: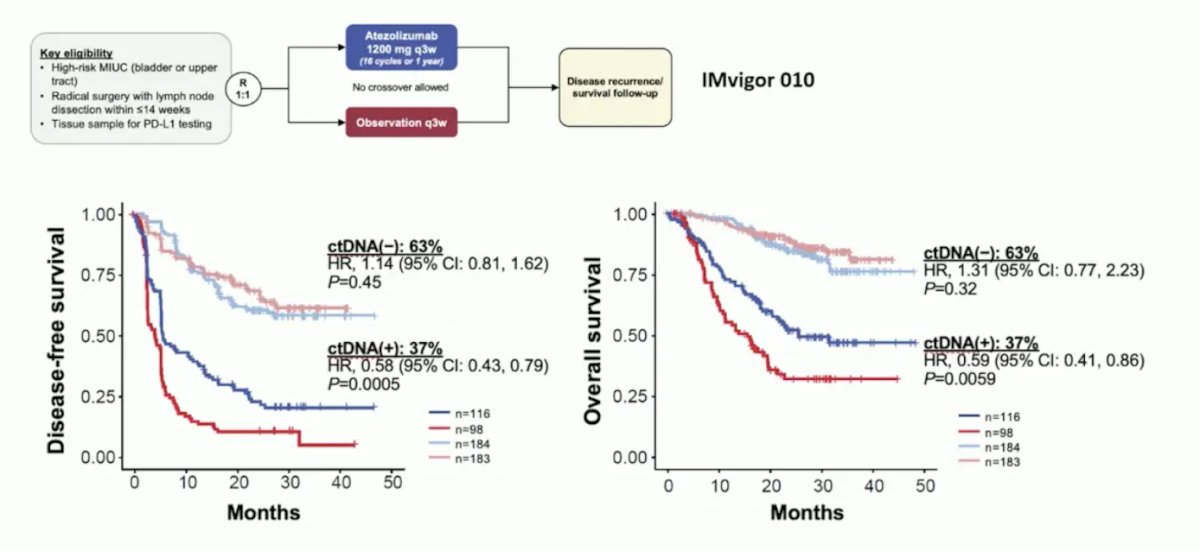
For both neoadjuvant chemotherapy followed by cystectomy and for neoadjuvant atezolizumab followed by cystectomy (ABACUS), ctDNA was useful for monitoring response to therapy: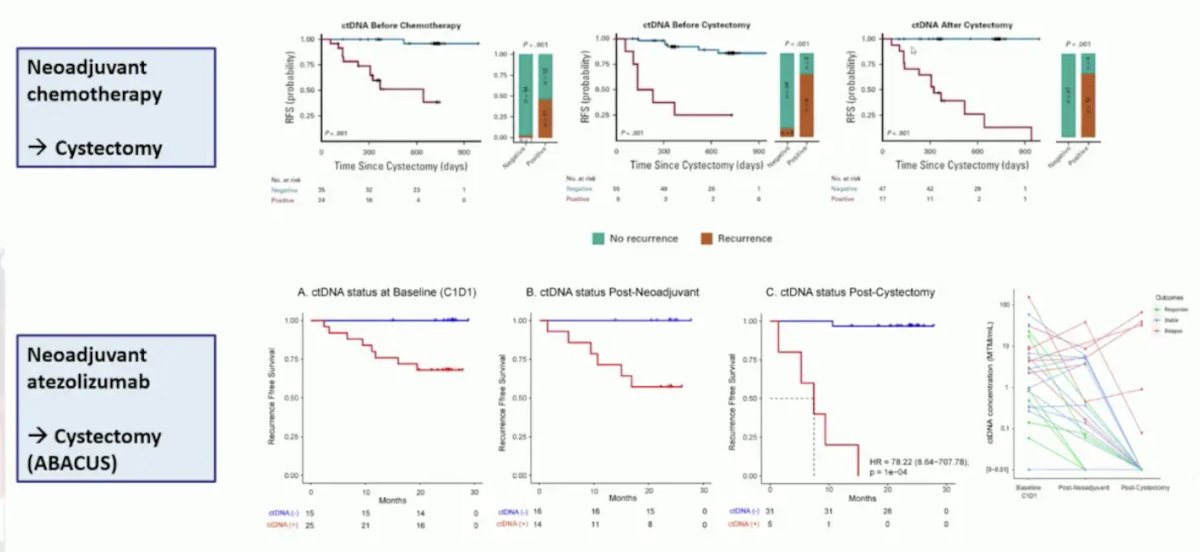
Dr. Szabados then discussed HER-2, which has been shown to correlate with worse outcomes in urothelial carcinoma when high HER-2 expression is noted. As such, the antibody-drug conjugate targeting HER-2, disitamab vedotin, has been shown to demonstrate a 52% objective response rate as a monotherapy, and a 76% objective response rate when combined with toripalimab: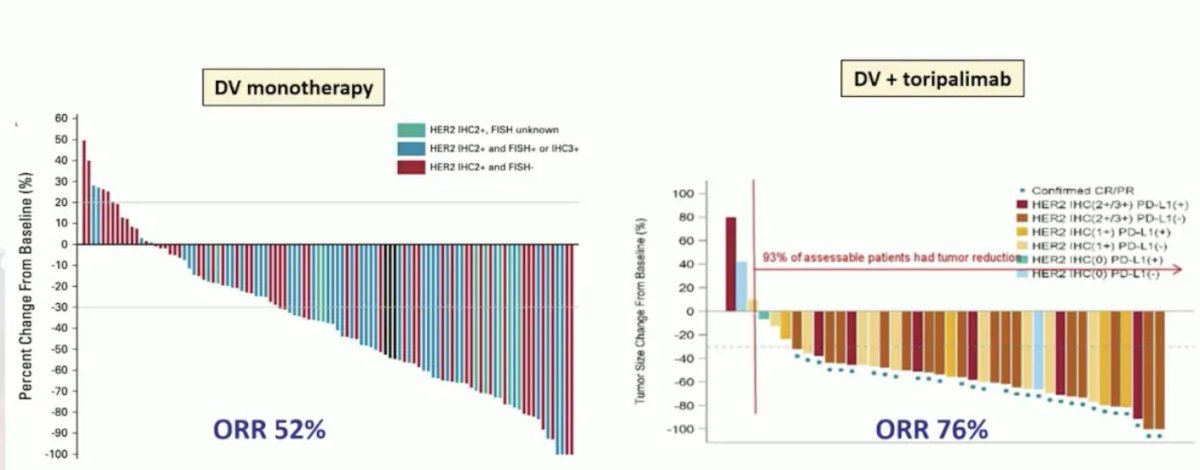
We await the preliminary results of RC48G001 Cohort C, which will be presented at this meeting. Dr. Szabados notes that via a press release on April 6, 2024, trastuzumab deruxtecan was approved for tumor-agnostic HER2-directed therapy for previously treated patients with metastatic HER2-positive solid tumors.
For enfortumab vedotin, results from EV-103 cohort K showed activity regardless of Nectin-4 expression, and we also await the exploratory analysis of EV-302 assessing nectin-4 expression to be presented at ESMO 2024. Finally, Trop-2 expression in sacituzumab govitecan-treated patients is broadly expressed and does not specifically correlate with outcomes.
For renal cell carcinoma, there are no established biomarkers, but there are several experimental (PD-L1, tumor mutational burden, RNA-based molecular subtypes, KIM-1, ctDNA, and CAIX) biomarkers. PD-L1 expression predicts higher benefit in IO-IO trials, including CheckMate 214 assessing first-line nivolumab + ipilimumab in first-line metastatic RCC, as well as in the nonclear cell RCC space based on data from SUNNIFORECAST presented at ESMO 2024. The utilization of PD-L1 in a clinical trial design is being further evaluated in the CARE-1 trial: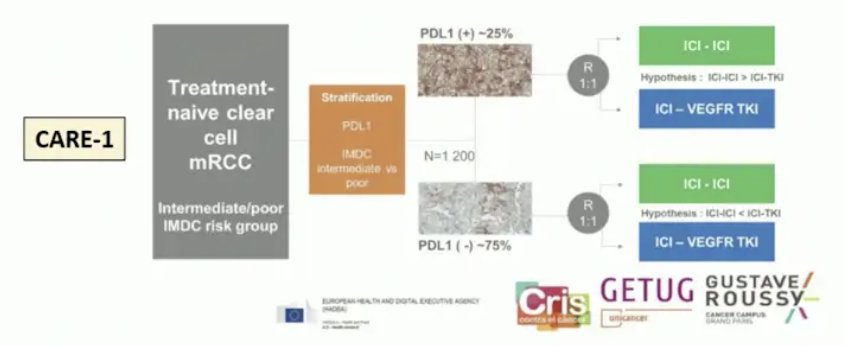
Molecular subtypes in RCC have shown some benefit, mainly angiogenesis high and Teff high signatures: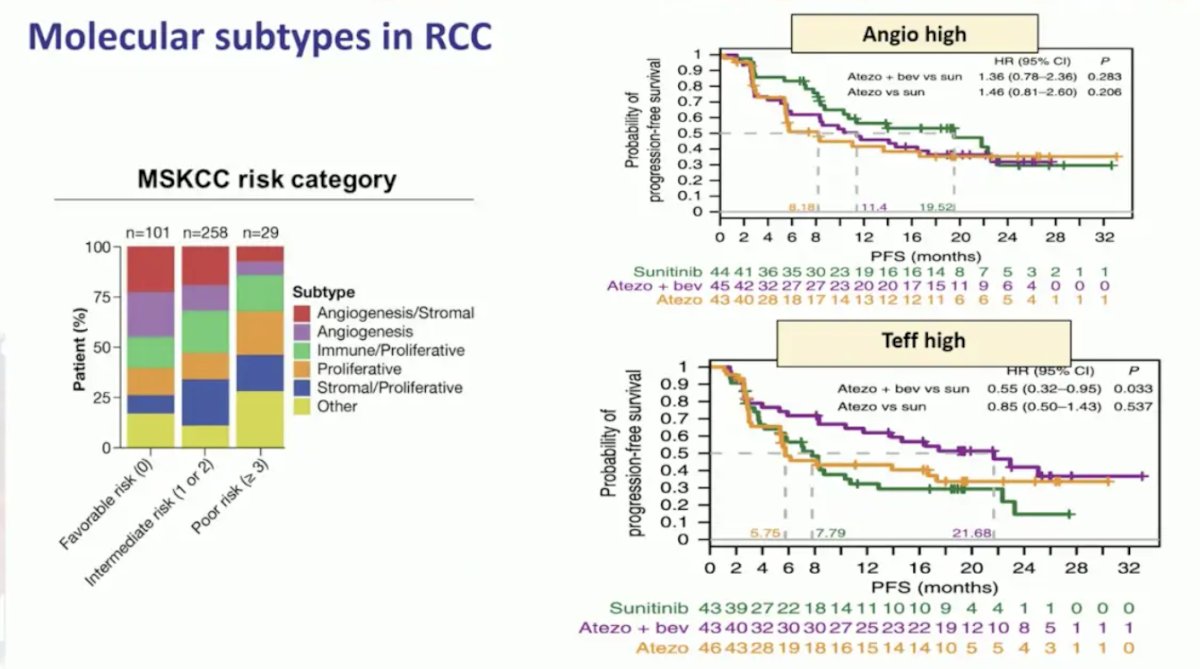
These molecular subtypes will be prospectively evaluated in the OPTIC RCC trial led by Dr. Brian Rini: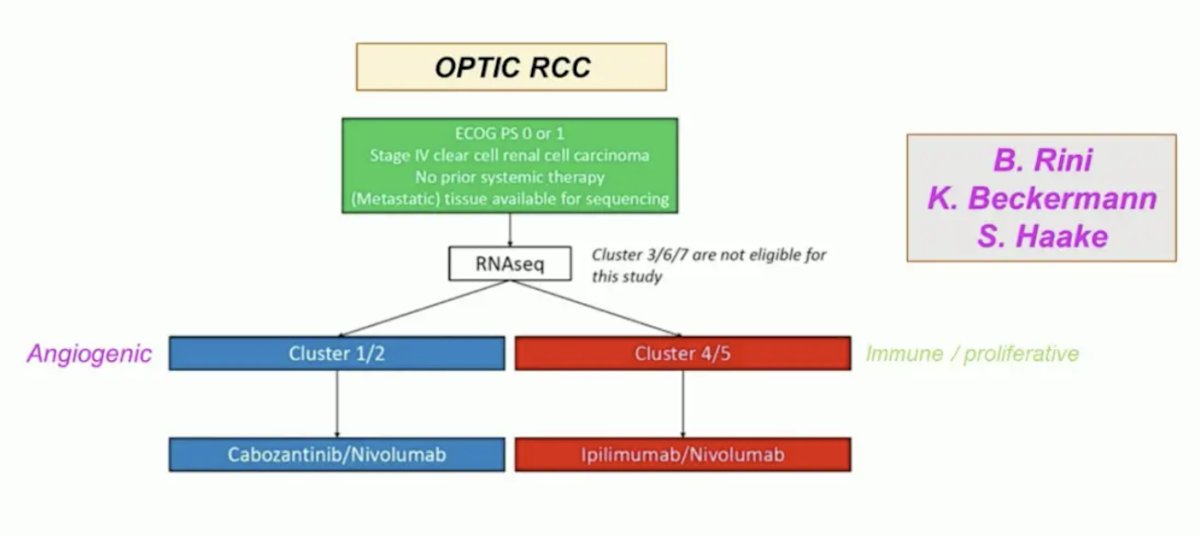
Presented initially at ASCO 2024, high baseline and on-treatment circulating KIM-1 is associated with worse outcomes in patients with RCC based on analysis of the IMmotion010 trial. KIM-1–high status was associated with reduced disease-free survival, and patients with KIM-1 high had better disease-free survival with atezolizumab versus placebo (HR 1.75, 95% CI 1.40-2.17):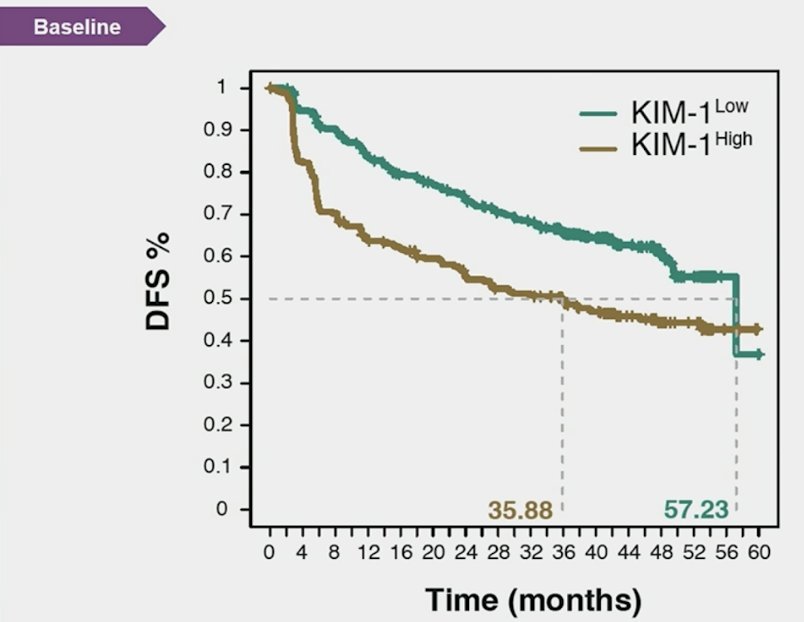
A ≥30% KIM-1 increase was associated with worse disease-free survival in both KIM1–high (atezolizumab HR 1.68, 95% CI 0.77, 3.69; placebo HR 3.53, 95% CI 2.24, 5.58) and KIM-1–low (atezolizumab HR 3.56, 95% CI 2.21, 5.75; placebo HR 3.22, 95% CI 1.81, 5.70) subgroups: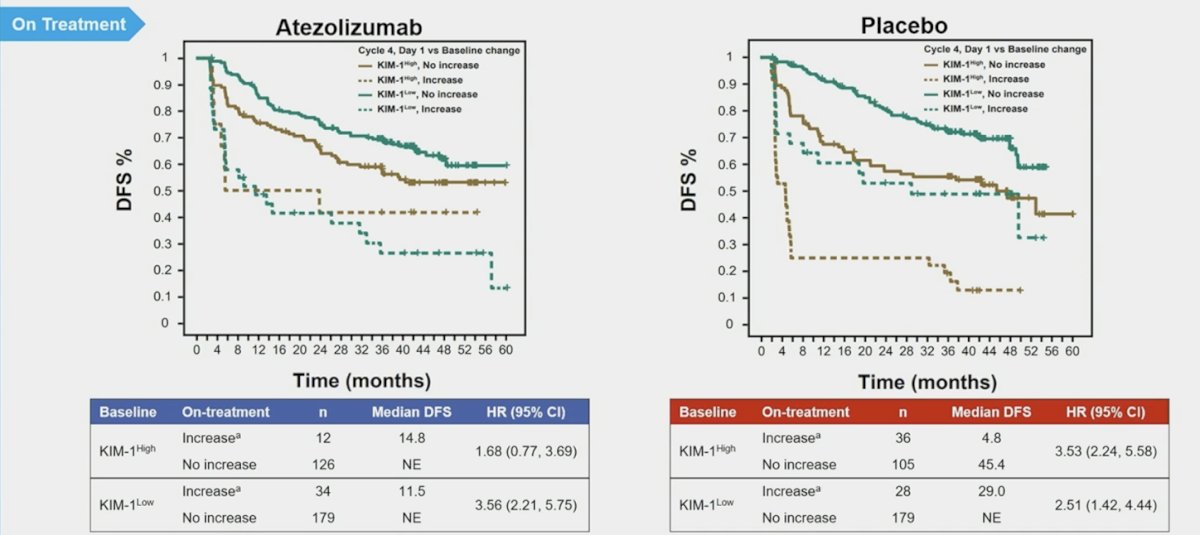
In patients with matched electrochemiluminescence samples (n = 103), median KIM-1 levels were higher (p < 0.001) at recurrence (172 pg/mL) than at baseline (79 pg/mL):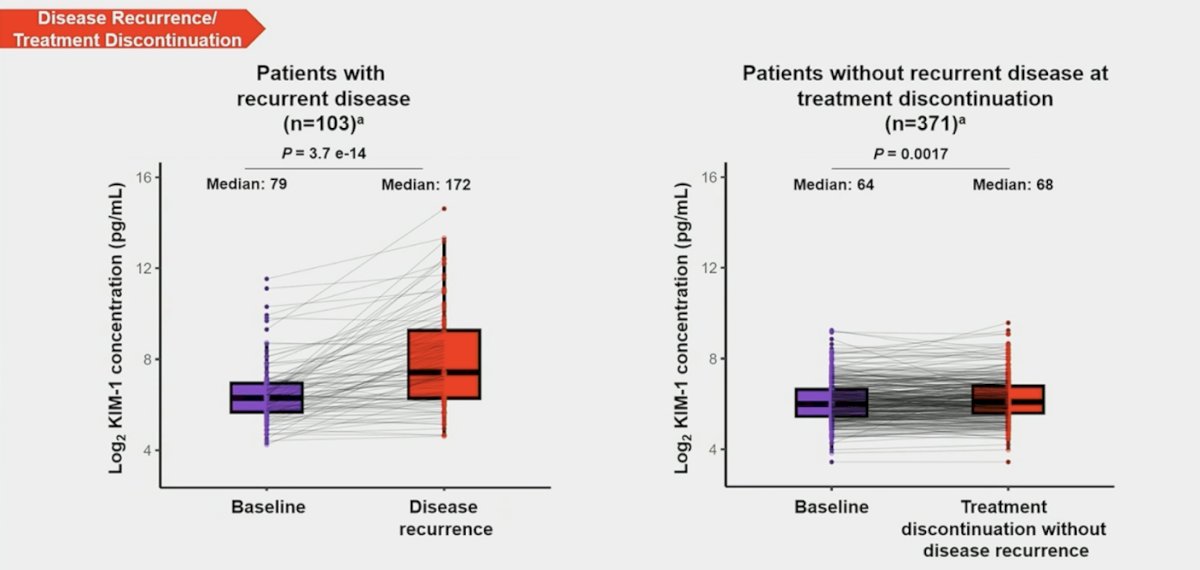
Finally, Dr. Szabados notes that girentuximab is a highly accurate imaging biomarker for clear cell RCC. CAIX is a cell surface transmembrane protein induced by hypoxia, and with hypoxia or VHL loss (~90% of clear cell RCC), CAIX is upregulated. Importantly, CAIX is minimally expressed in normal tissue.
Dr. Szabados concluded her presentation discussing circulating versus tissue-based biomarkers with the following take-home points:
- For urothelial carcinoma:
- The early part of biomarker discovery has been a mixture of failures and successes
- Immunotherapy-related biomarkers remain elusive
- Biomarkers for targeted therapies (ie. FGFR, HER2) look more promising but are not perfect
- Circulating biomarkers also look very attractive
- For renal cell carcinoma:
- Biomarkers for VEGF therapy have never really been developed in the past
- Now, we have angiogenetic signatures, which appear more reliable for VEGF-targeted therapy than for immunotherapy
- The jury is still out on PDL1 and its role
- Circulating biomarkers appear more complicated in RCC (lower ctDNA shedding), but KIM-1 looks promising
Presented by: Bernadett E. Szabados, MD, Urologist, Barts Cancer Institute, Queen Mary University of London, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021 Jl;595(7867):432-437.


