(UroToday.com) The 2024 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Joshua Meeks discussing a prospective evaluation of BCG unresponsive bladder cancer carcinoma in situ (CIS) to identify genetic mechanisms of immunotherapy resistance and targeted therapy using an ultra-sensitive next-generation sequencing minimal residual disease assay.
While there are multiple emerging treatments for BCG unresponsive CIS bladder cancer, the early detection of recurrence and targeting of immunotherapy resistance is a significant unmet need. To date, the genomic profile of BCG unresponsive CIS has not been described. Therefore, developing ultra-sensitive, bespoke, urine-based genomic assays could transform bladder cancer surveillance and identify new therapeutic strategies. Dr. Meeks and colleagues performed a prospective profiling of BCG unresponsive CIS and described the early response to salvage therapy.
Patients with BCG unresponsive CIS were prospectively enrolled before starting salvage therapy. Urine samples were collected at baseline and follow-up time points. All sequencing was performed using the PredicineBEACON minimal residual disease assay, including WES of 20,000 genes and boosted sequencing of 600 cancer genes at baseline using tissue and/or urine. Bespoke personalized minimal residual disease panels include 16-50 personalized mutations and 500 hotspot mutations. Mutations were annotated, and allele frequency was calculated. FFPE samples were also tested with PredicineWES+ tissue assay at baseline when the samples were available. Minimal residual disease was positive if two or more mutations were detected. An overview of the patients and tests is as follows: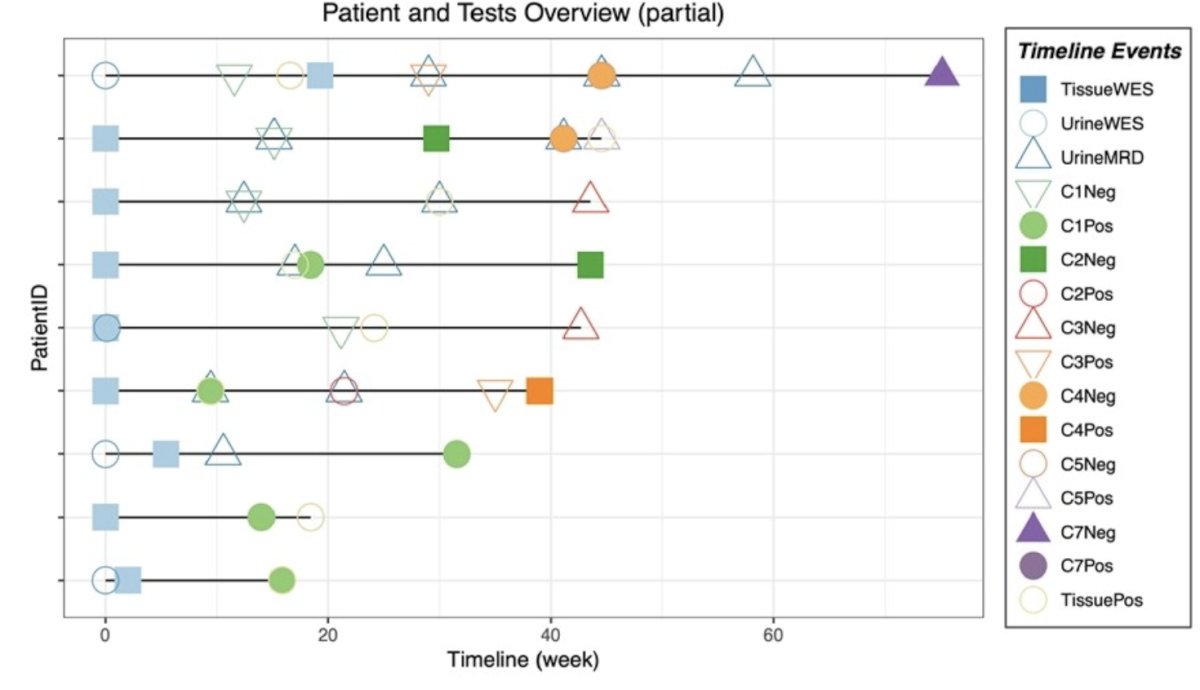
The following oncoplot summarized variant, CNV, and fusion findings in the baseline samples (FFPE and/or urine) tested with PredicineWES+: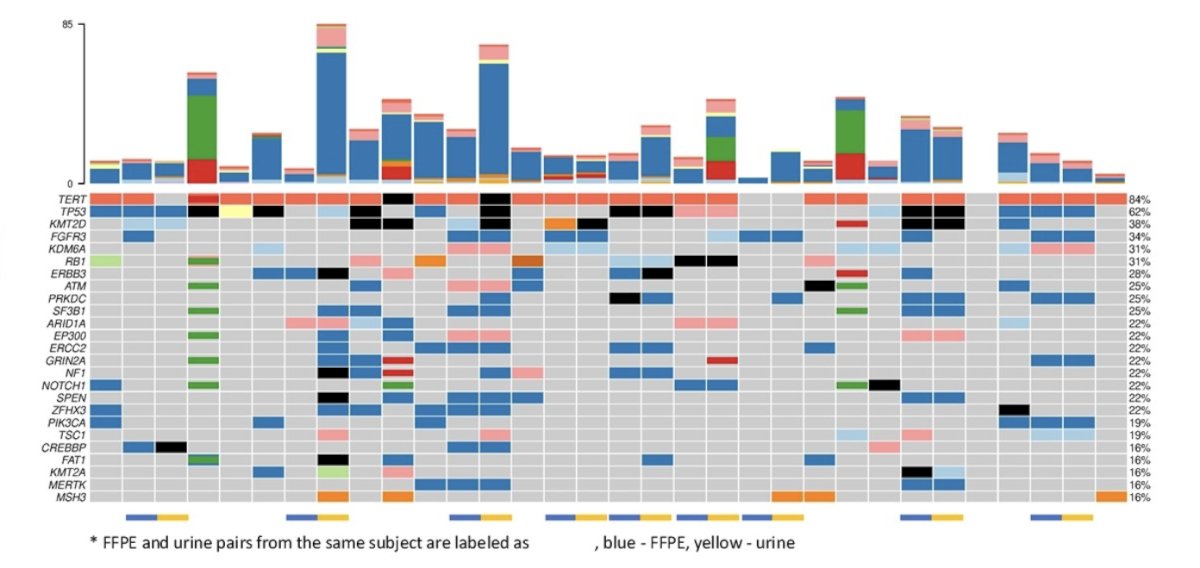
FFPE and urine pairs were indicated by the label at the bottom and at the top, the 25 most frequently identified genes in these samples were shown, with the occurrence noted at the right margin. Tumor fraction changes were compared to the baseline sample and along the time course. The minimal residual disease assay status was determined by whether the minimal residual disease samples had two or more tracking mutations. The prevalence of minimal residual disease-positive status among the follow-up samples, even when the tumor fractions have significantly dropped, may have reflected the nature of CIS. However, one observation was that for all the samples that reached minimal residual disease negative status, the subjects showed stable tumor fraction in the following time points: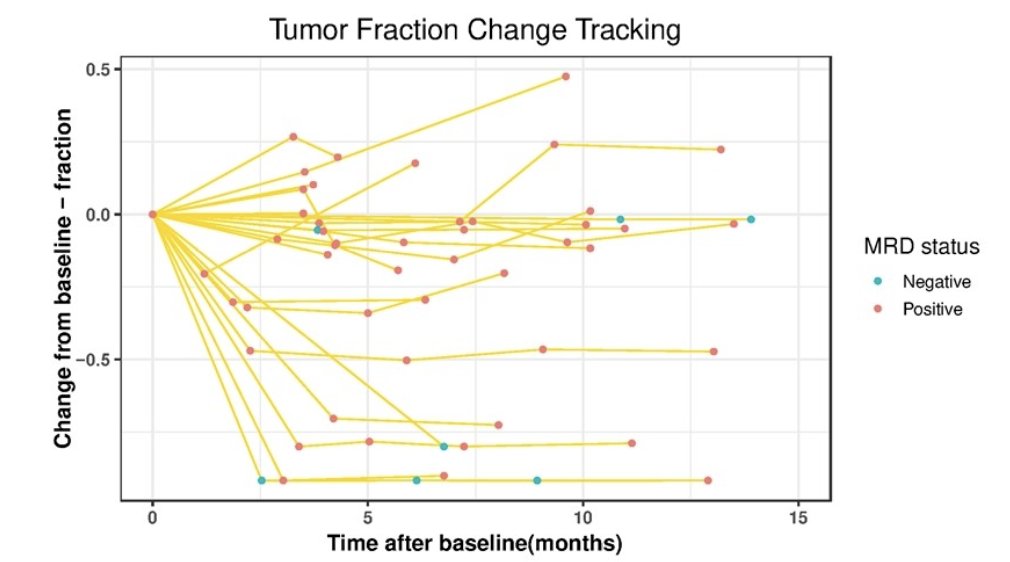
Below are two case studies of patients showing the follow-up tumor fraction values in detail. Patient 1 demonstrates that when the sample has reached minimal residual disease negative status, the samples maintained a low tumor fraction status, even though the minimal residual disease status may still fluctuate. Patient 2 developed a recurrence, with the tumor fraction surge observed in the urine and minimal residual disease test predating clinical confirmation by 6 months: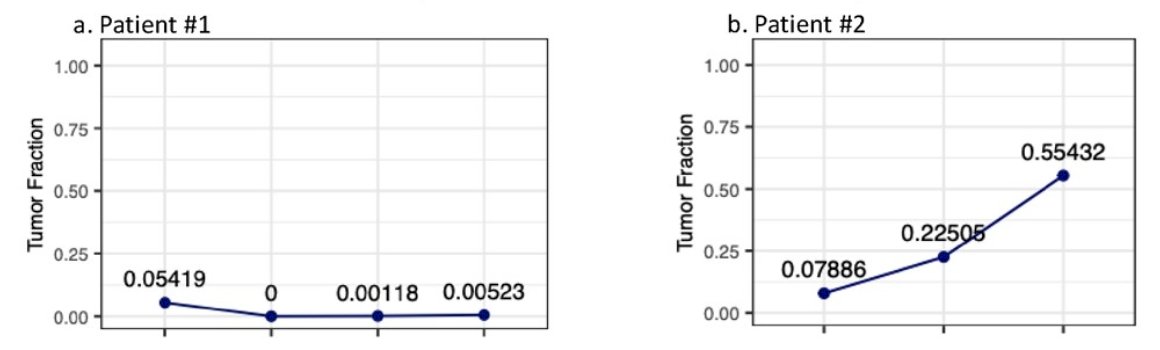
Among the 54 samples tested with a personalized minimal residual disease, 48/54 were minimal residual disease positive before salvage treatment. While 44/48 minimal residual disease positive had a decrease in tumor fraction at the first evaluation, only 4 converted to minimal residual disease negative at 3 months. Because all tumors were BCG unresponsive, the investigators evaluated for mutations associated with immunotherapy resistance.
The median urinary tumor mutational burden of baseline samples was 3.92 (range: 0.3 to 24.1), the median FFPE tumor mutational burden of baseline samples was 7.7 (range: 1.62 to 14.8). The overall tumor mutational burden score is also 3.92, suggesting an overlapping distribution of tumor mutational burden scores between urine and FFPE samples: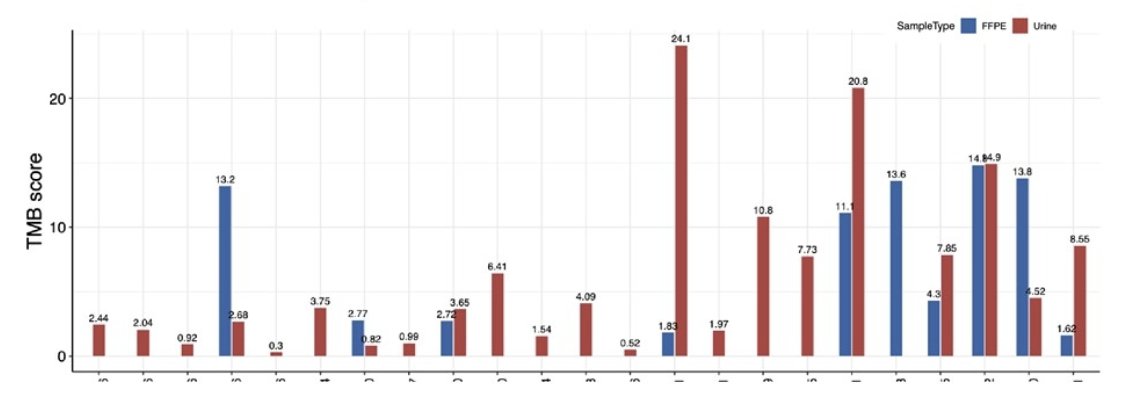
The most frequent mutations were TERT (84%), TP53 (62%), RB1 (31%), FGFR3 (34%), KMT2D (38%), and ERBB3 (28%). Dr. Meeks and colleagues evaluated for alterations in the PDL1 pathway and identified CNV changes in CD274(PDL1) or PDCD1LG2(PDL2) genes in 4/23 subjects. Mutations in the IFN-gamma pathway gene JAK2 were found in 3/23 (10% SNVs and 16% CNVs of JAK1/2). Of note, FGFR3 hotspots for bladder cancer, including p.S249C and p.G730C were found in 6/23 subjects.
Dr. Meeks concluded his presentation discussing a prospective evaluation of BCG unresponsive bladder cancer CIS to identify genetic mechanisms of immunotherapy resistance and targeted therapy using an ultra-sensitive next-generation sequencing minimal residual disease assay with the following take-home points:
- Nearly all patients with BCG unresponsive CIS begin salvage therapy with minimal residual disease positive and have a quantifiable decline in tumor fraction with treatment
- Genomic alterations were identified that were previously reported with immune escape and actionable mutations in DNA damage repair and the FGFR3 pathway, now targetable with intravesical therapy.
Presented by: Joshua Meeks, MD, PhD, Associate Professor, Northwestern University, Chicago, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


