(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Dr. Bradley McGregor presented updated efficacy results from the Double Antibody Drug conjugate (DAD) phase I trial of sacituzumab govitecan plus enfortumab vedotin in the ≥2nd line treatment setting for metastatic urothelial carcinoma patients.
Sacituzumab govitecan and enfortumab vedotin are antibody-drug conjugates used sequentially for the treatment of metastatic urothelial carcinoma patients. These agents have non-overlapping toxicities and potential synergistic mechanisms of action, which supports their combinatory evaluation in a clinical trial setting. Results of the phase I study evaluating the safety and maximum tolerated dose of the combination of sacituzumab govitecan plus enfortumab vedotin in patients with treatment-resistant metastatic urothelial carcinoma have previously been published (NCT04724018).1 In this report, Dr. McGregor presented the safety and efficacy outcomes after 22 months of follow-up.
Patients with metastatic urothelial carcinoma and Eastern Cooperative Oncology Group (ECOG) performance status ≤1 who had progressed on platinum and/or immunotherapy were enrolled. Sacituzumab govitecan and enfortumab vedotin were administered on days 1 + 8 of a 21-day cycle until progression or unacceptable toxicity. The primary endpoint was safety, as assessed by maximum tolerated dose of this combination through Bayesian Optimal Interval Design. The secondary endpoints were objective response rate (ORR), progression-free survival, and overall survival.
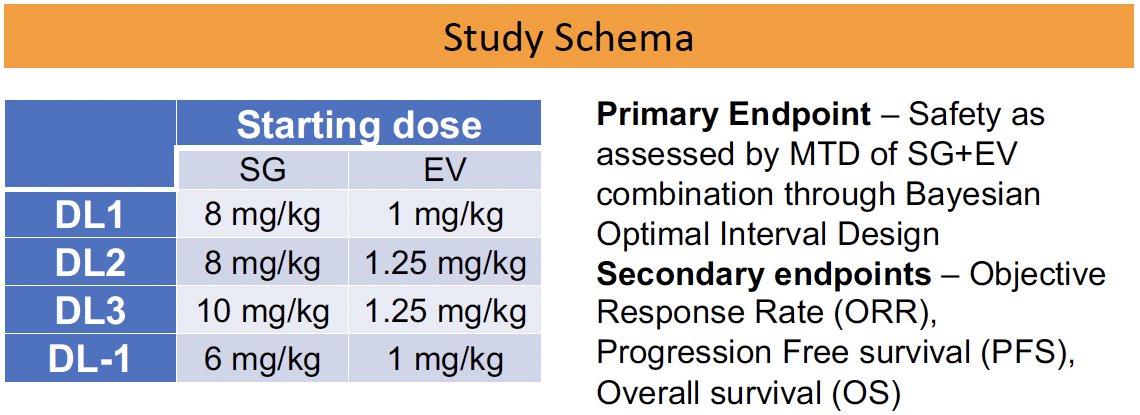
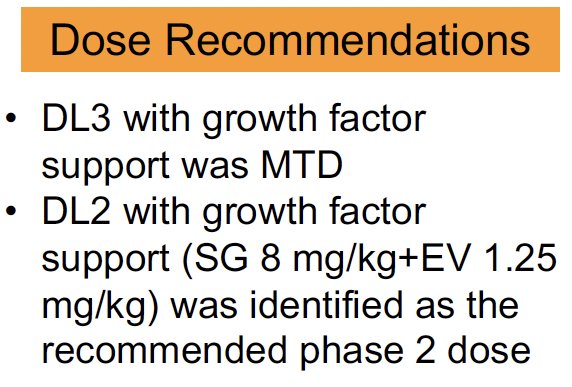
The dose recommendations are as follows:
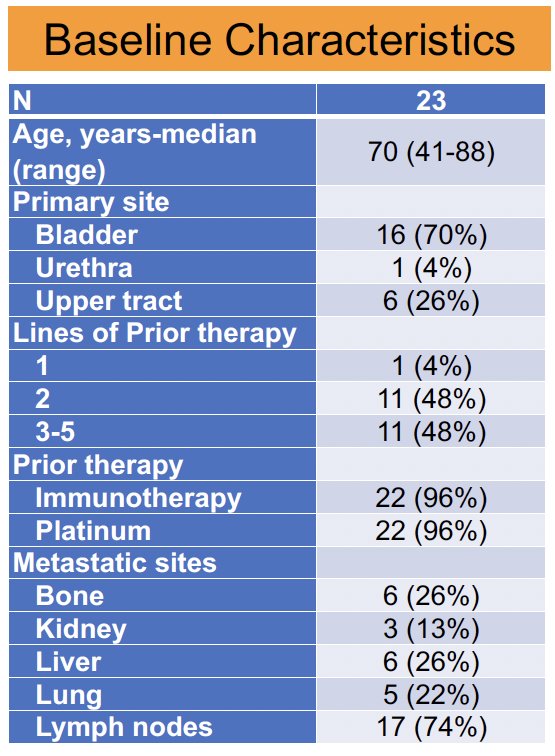
The baseline patient characteristics are summarized below (n=23). The median patient age was 70 years. The primary tumor site was bladder for 70% of patients. Almost 50% of patients had received 2 prior lines of therapy, and an additional 50% had received 3–5 prior lines of therapy. Almost all patients had received prior immunotherapy and platinum-based combination chemotherapy.
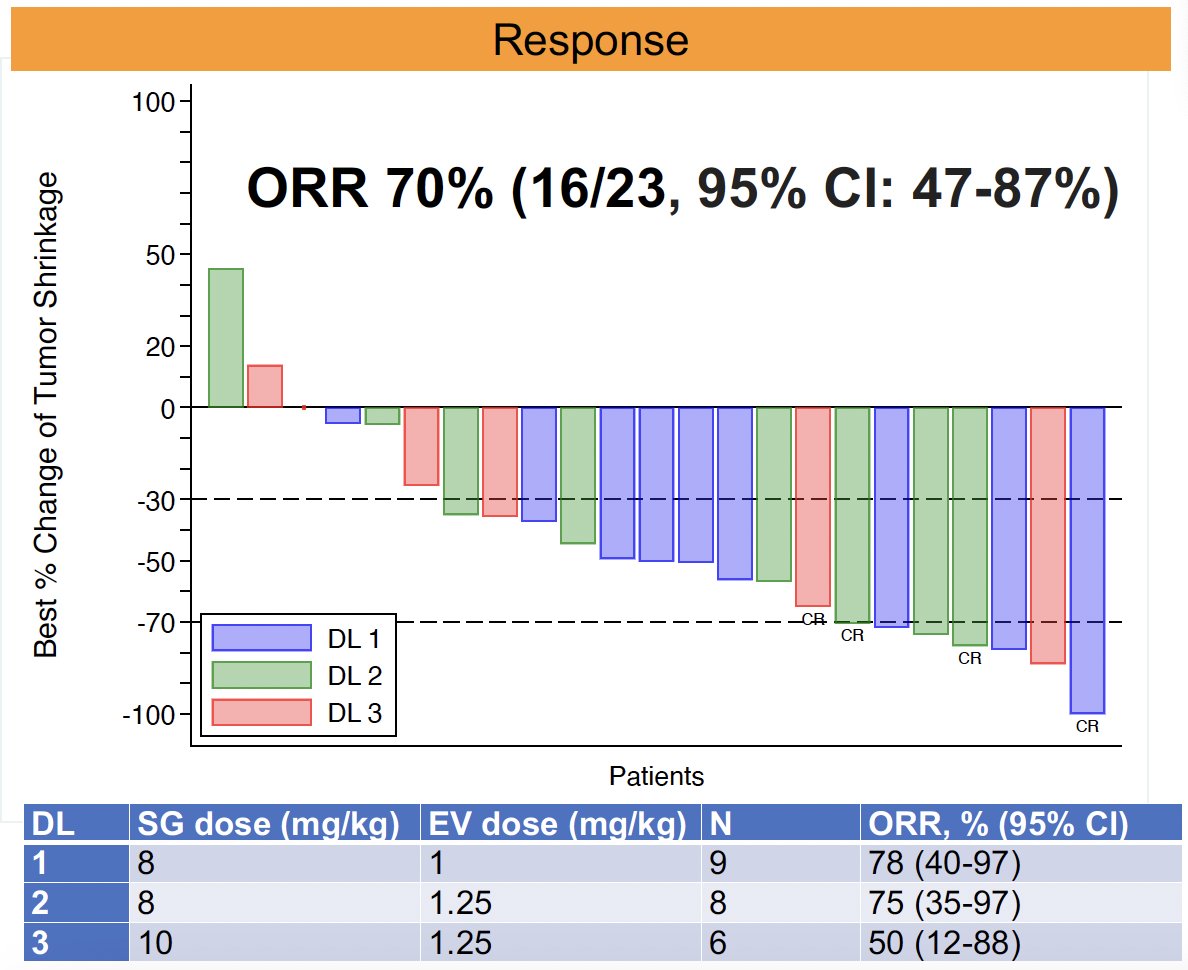
The objective response rate of this combination was 70% (16/23; 95% CI: 47–87%). Of the 16 responders, 4 had a complete response and 12 had a partial response. The median duration of response was 10 months, with ongoing response in 6 patients.
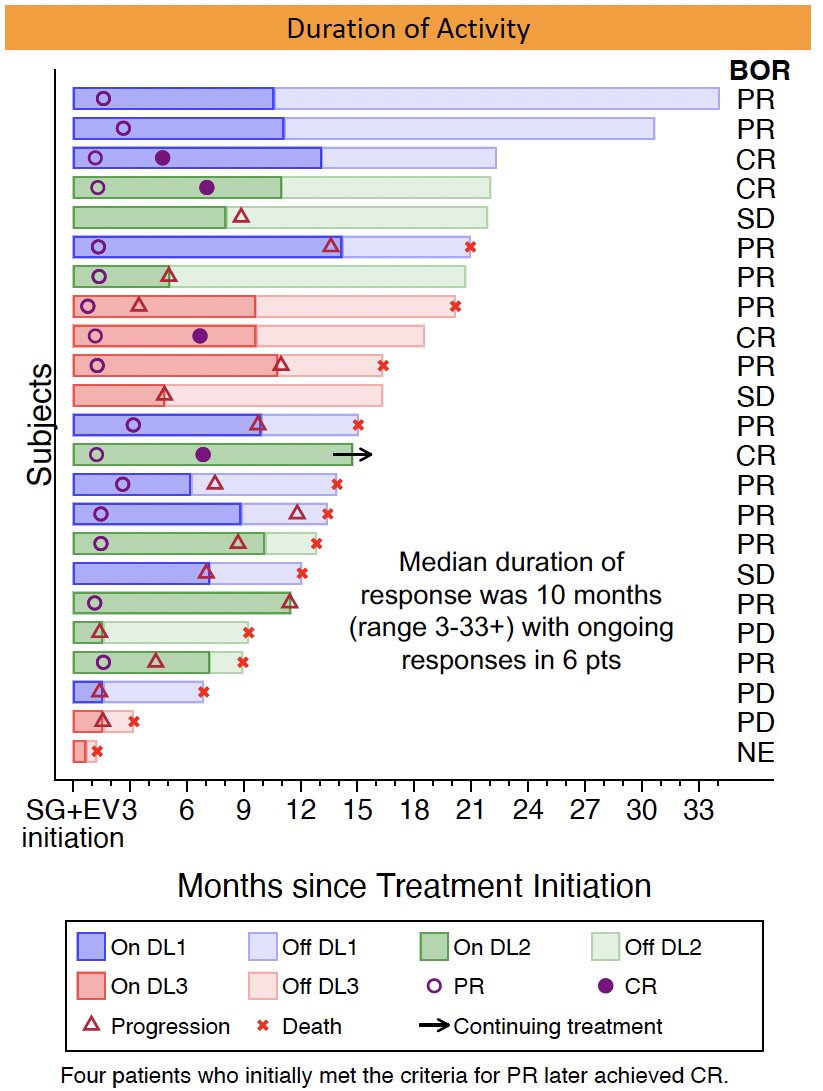
There were no new safety signals or unexpected delayed toxicities. 15/23 patients received prophylactic G-CSF support with cycle 1.
Dr. McGregor concluded as follows:
- With a median follow-up of 22 months, the combination of sacituzumab govitecan and enfortumab vedotin continues to show encouraging activity with an ORR of 70% and no new safety signals in patients with treatment resistant metastatic urothelial carcinoma.
- This data supports ongoing expansion cohorts of sacituzumab govitecan 7.5 mg/kg and enfortumab vedotin 1.25 mg/kg on days 1 and 8 every 21 days in the treatment-resistant setting and in combination with pembrolizumab as first line therapy for metastatic urothelial carcinoma (NCT04724018)
Presented by: Bradley A. McGregor, MD, Director of Clinical Research, Lank Center of Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- McGregor BA, Sonpavde GP, Kwak L, et al. The Double Antibody Drug Conjugate (DAD) phase I trial: sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann Oncol. 2024; 35(1):91–7.


