(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the poster 1968 presentation. Dr. Jonathan E. Rosenberg presented the results of the EV-103 Study with five-year follow-up of first line Enfortumab Vedotin (EV) + Pembrolizumab in Cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC).
Dr. Rosenberg began his presentation by noting that, until recently, the first-line treatment for locally advanced or metastatic urothelial carcinoma (la/mUC) was determined by cisplatin eligibility. Unfortunately, approximately half of the patients with la/mUC are ineligible for cisplatin due to impaired renal function, poor performance status, and other comorbidities, which limits their systemic treatment options.
The EV-302/KEYNOTE-A39 trial (NCT04223856), a Phase 3 study evaluating enfortumab vedotin (EV) in combination with pembrolizumab versus chemotherapy, led to the recent FDA approval of EV plus pembrolizumab as a first-line treatment option for la/mUC. This trial demonstrated, for the first time, an overall survival (OS) benefit compared to platinum-based chemotherapy in patients with previously untreated la/mUC, regardless of cisplatin eligibility.1 Since then, EV plus pembrolizumab has been included in several treatment guidelines.
The dose escalation and Cohort A of the ongoing phase 1b/2 multicohort study EV-103 (NCT03288545) are assessing EV in combination with pembrolizumab in cisplatin-ineligible patients with previously untreated la/mUC. In this poster presentation, Dr. Rosenberg reported the updated efficacy and safety data after 5 years of follow-up for Cohort A of EV-103. The study design is illustrated below.

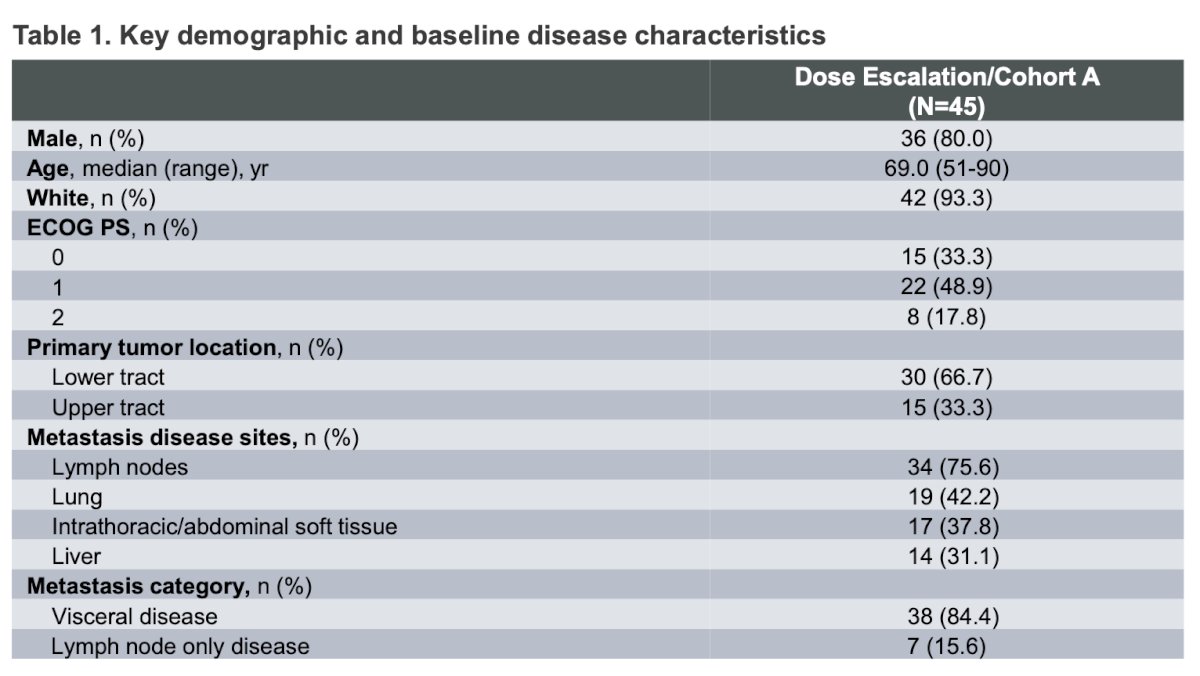
Dr. Rosenberg mentioned that the baseline patient characteristics were representative of the cisplatin-ineligible population with la/mUC, consistent with what has been observed in other trials. Briefly, 84% of patients had visceral disease, 17.8% had an ECOG-PS of 2, and one-third of the tumors were located in the upper tract.
At the time of data cutoff (median follow-up of 62 months), 33% of patients remained on the study. Patients received a median of 7 cycles of EV plus pembrolizumab, with a median treatment duration of 7 months. The patient disposition at the time of data cutoff is illustrated in the table below.

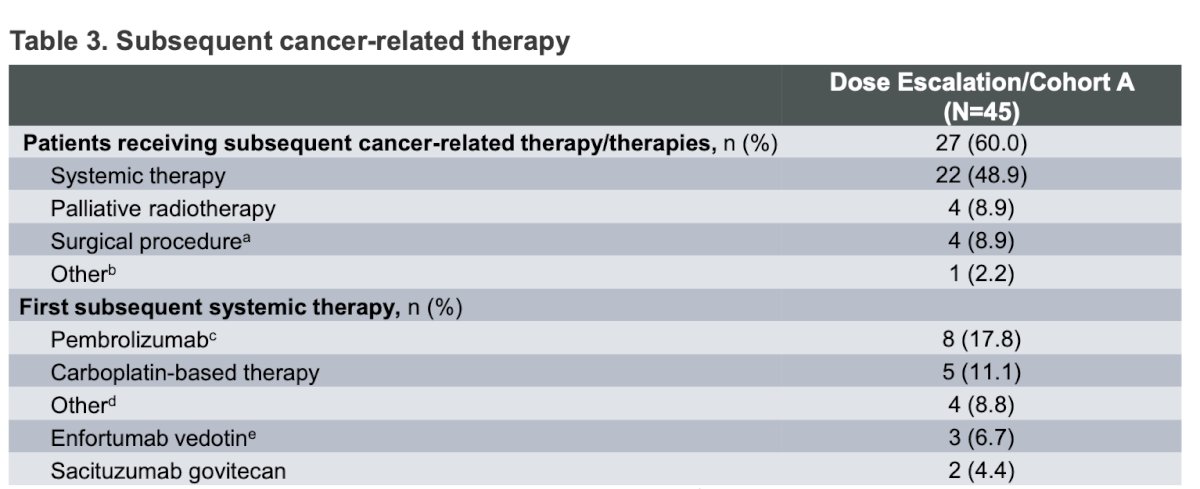
Dr. Rosenberg pointed out that after discontinuation of the study treatment, 60% of patients received subsequent cancer-related therapy. Interestingly, the most frequent type of subsequent systemic therapy was pembrolizumab in 17.8% of patients, followed by carboplatin-based chemotherapy in 11%.
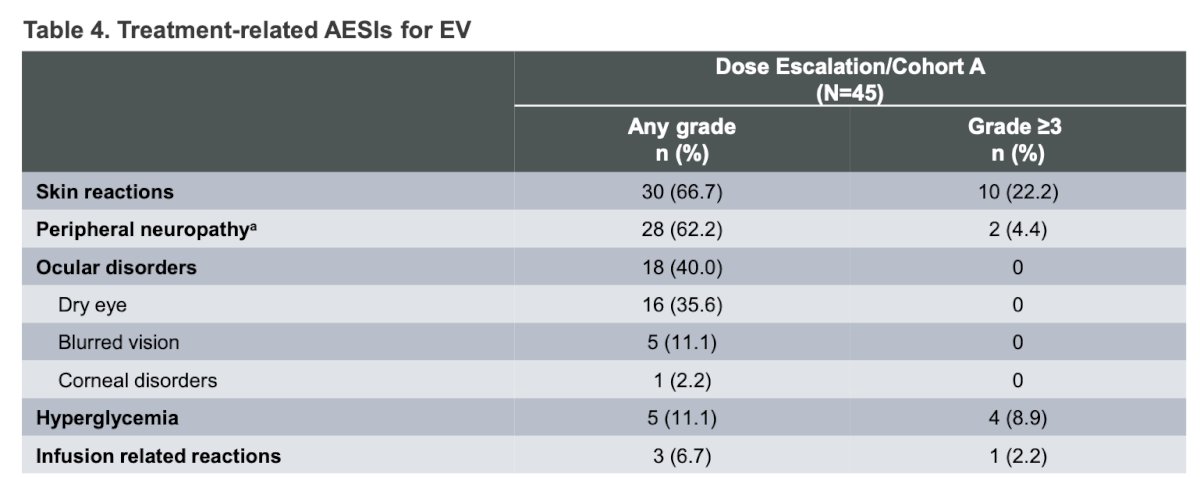
The most common treatment-related adverse events (TRAEs) for EV were skin reactions (67%), followed by peripheral neuropathy (62%). The most common Grade ≥3 TRAEs were skin reactions (22.2%), followed by hyperglycemia (8.9%). Other TRAEs are depicted in the table below.
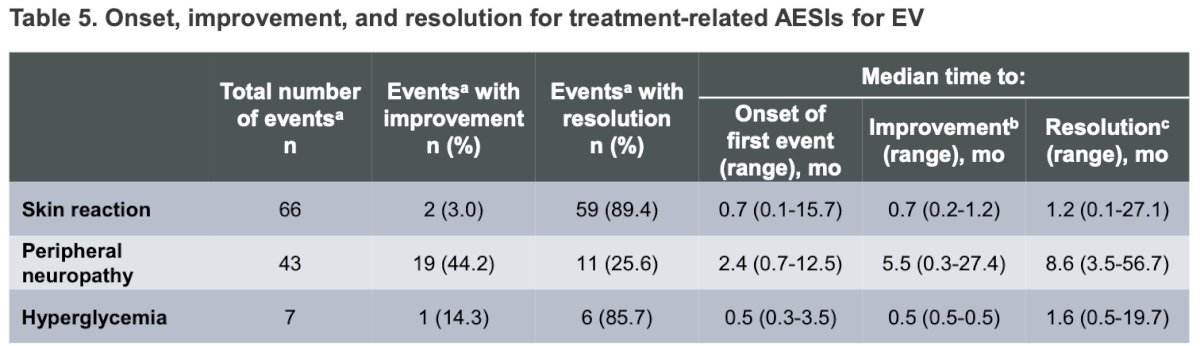
Fortunately, the majority of EV-related TRAEs improved or resolved after therapy discontinuation. Among patients with skin reactions, 89.4% experienced complete resolution. For those with peripheral neuropathy, 25.6% had complete resolution, while 44% saw improvement.
The confirmed overall response rate (ORR) for EV plus pembrolizumab was 73.3%, with 15.6% of patients achieving a complete response and 57.8% a partial response. The disease control rate (DCR) was 84.4% at the time of data cutoff.
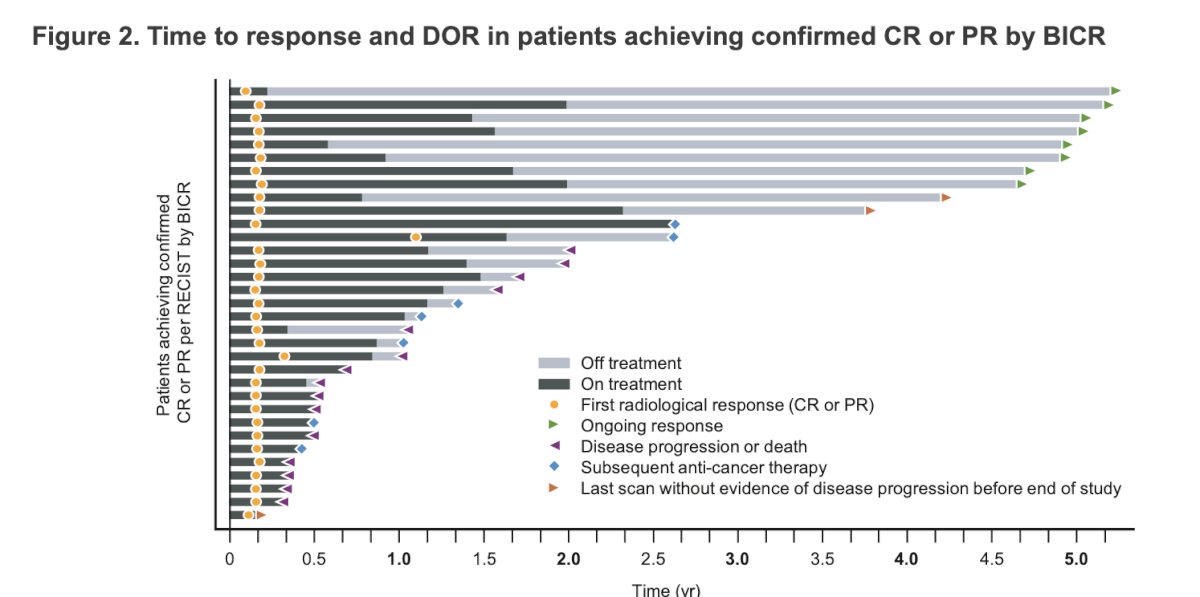
The median duration of response (DOR) was 22.1 months, and the probability of responders without progressive disease or death plateaued at 47% after 1.8 years.
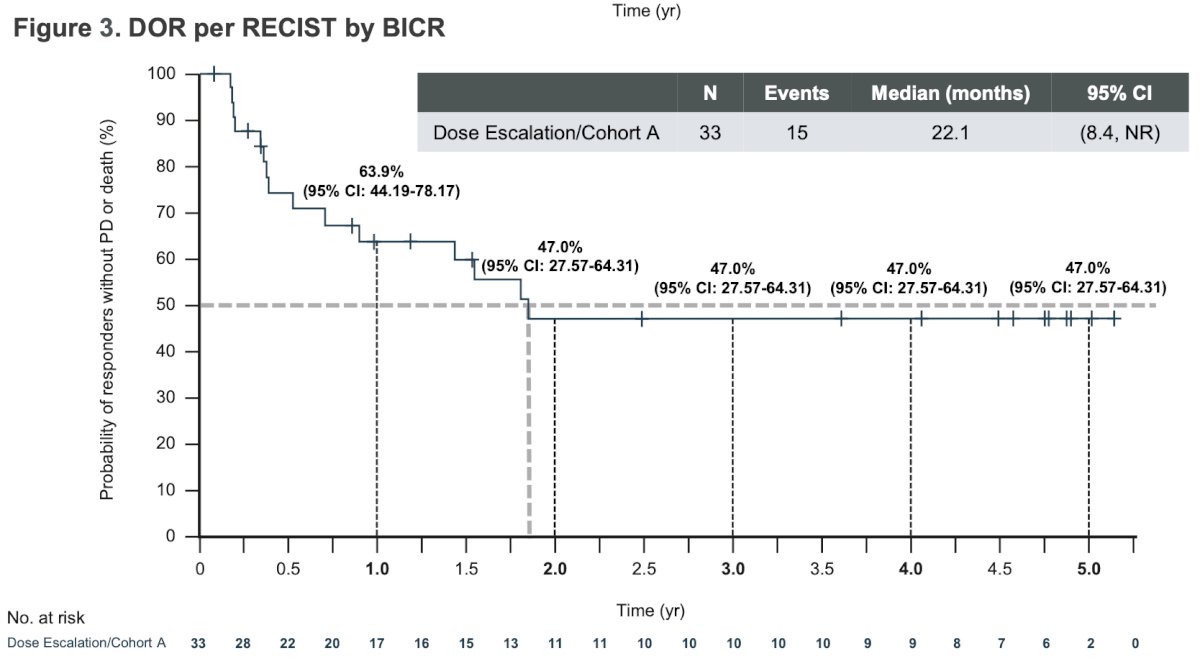
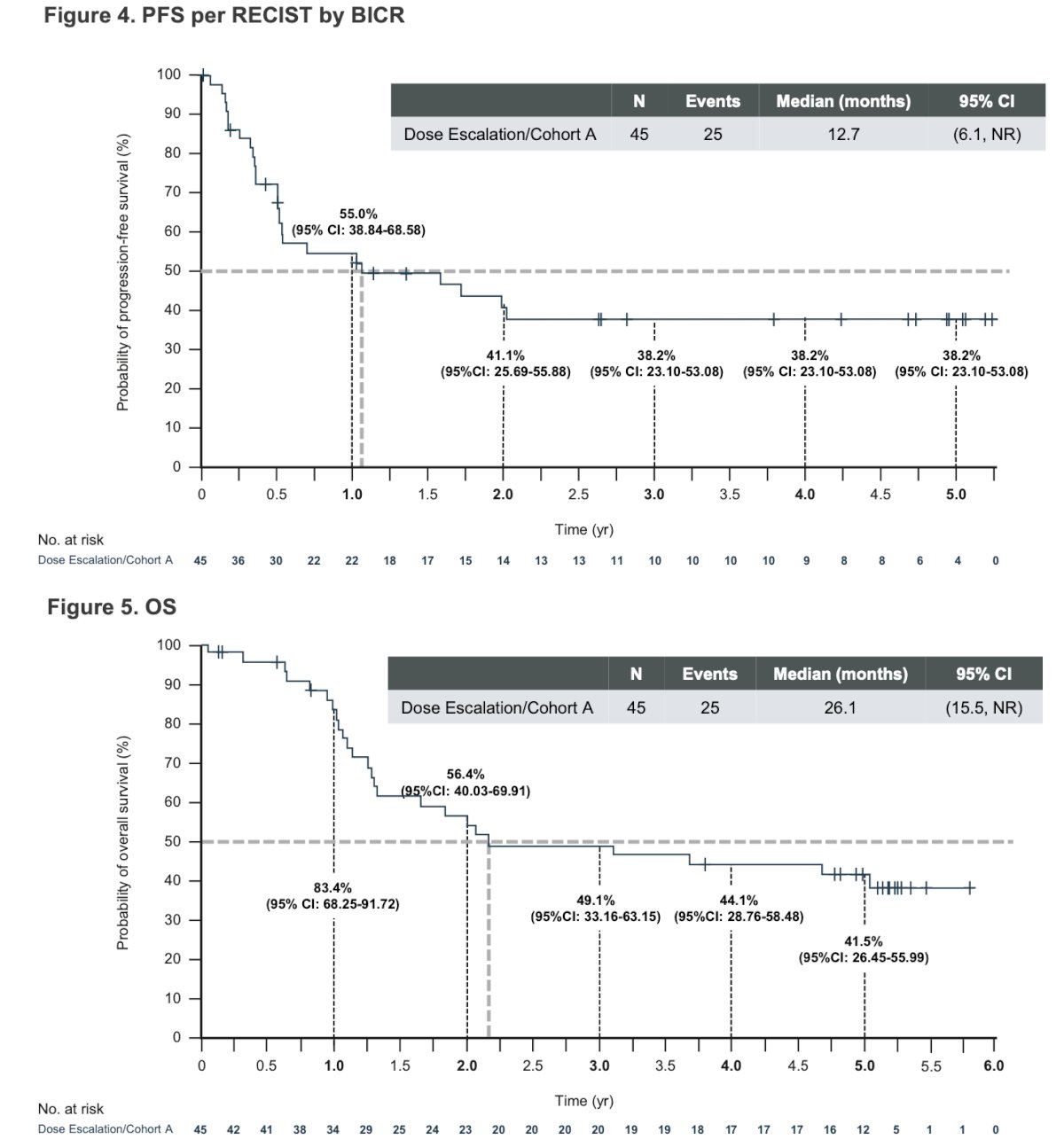
The median progression free survival was 12.7 months and the median OS was 26.1 months.
Dr Rosenberg concluded his poster presentation with the following messages:
- After 5 years of follow-up, EV plus pembrolizumab in the first line setting for patients with cisplatin-ineligible la/mUC demonstrated durable responses and meaningful survival outcomes, with a median overall survival (OS) of 26 months.
- An unprecedented 41.5% of patients treated with EV plus pembrolizumab were alive at 5 years, surpassing historical data from the phase 2/3 EORTC 30986 study (GC vs. M-CAVI)
- These results, along with data from the EV-302 study, further support the use of EV plus pembrolizumab as the first-line standard of care for patients with la/mUC, regardless of cisplatin eligibility.
Presented by: Jonathan E. Rosenberg, MD, Genitourinary Oncologist at Memorial Sloan Kettering Cancer Center
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.
- De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, Collette S, Lorent J, de Wit R, Sylvester R. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012 Jan 10;30(2):191-9. doi: 10.1200/JCO.2011.37.3571. Epub 2011 Dec 12. PMID: 22162575; PMCID: PMC3255563.


