(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the Poster presentation of poster 1988. Dr. Tanya Jindal presented on behalf of the UNITE study collaborators, exploring Enfortumab vedotin (EV) + pembrolizumab (P) outcomes outside clinical trials and biomarkers of benefit in patients with advanced urothelial carcinoma (aUC).
The first-line combination therapy of Enfortumab vedotin and Pembrolizumab has demonstrated improved outcomes compared to platinum-based chemotherapy in the EV-302 trial and has become the standard of care for aUC. However, there is a need for data on biomarkers and real-world outcomes associated with this treatment regimen.
UNITE is a retrospective cohort study focusing on patients with aUC, specifically examining clinical outcomes for those treated with novel targeted agents like Enfortumab vedotin. This collaboration spans several academic sites across the United States. Patients who were treated with Enfortumab vedotin and Pembrolizumab were identified from the UNITE collaboration and included in this study.
Biomarkers were assessed in all patients treated with Enfortumab vedotin and Pembrolizumab for whom data were available. The biomarkers included tumor mutational burden (TMB) and genomic alterations from next-generation sequencing (NGS) that were present in ≥10% of patients. Each potential biomarker’s impact on clinical outcomes was evaluated using univariate analysis, while multivariate analysis was used to adjust for confounding clinical variables. Patients treated within clinical trials were excluded from time-to-event endpoints and objective response rate (ORR) assessments.
The time-to-event endpoints of this analysis included:
- Progression-free survival (PFS)
-Overall survival (OS)
The investigators identified 189 patients treated with Enfortumab vedotin and Pembrolizumab across 14 U.S. sites in the UNITE study. Of these, 171 patients received treatment outside of clinical trials, and NGS data were available for 118 patients. Baseline and demographic characteristics are illustrated below. Briefly, most patients had pure urothelial carcinoma (62%), had received fewer than 2 prior lines of therapy (88%), and had their primary tumor located in the lower tract (78%).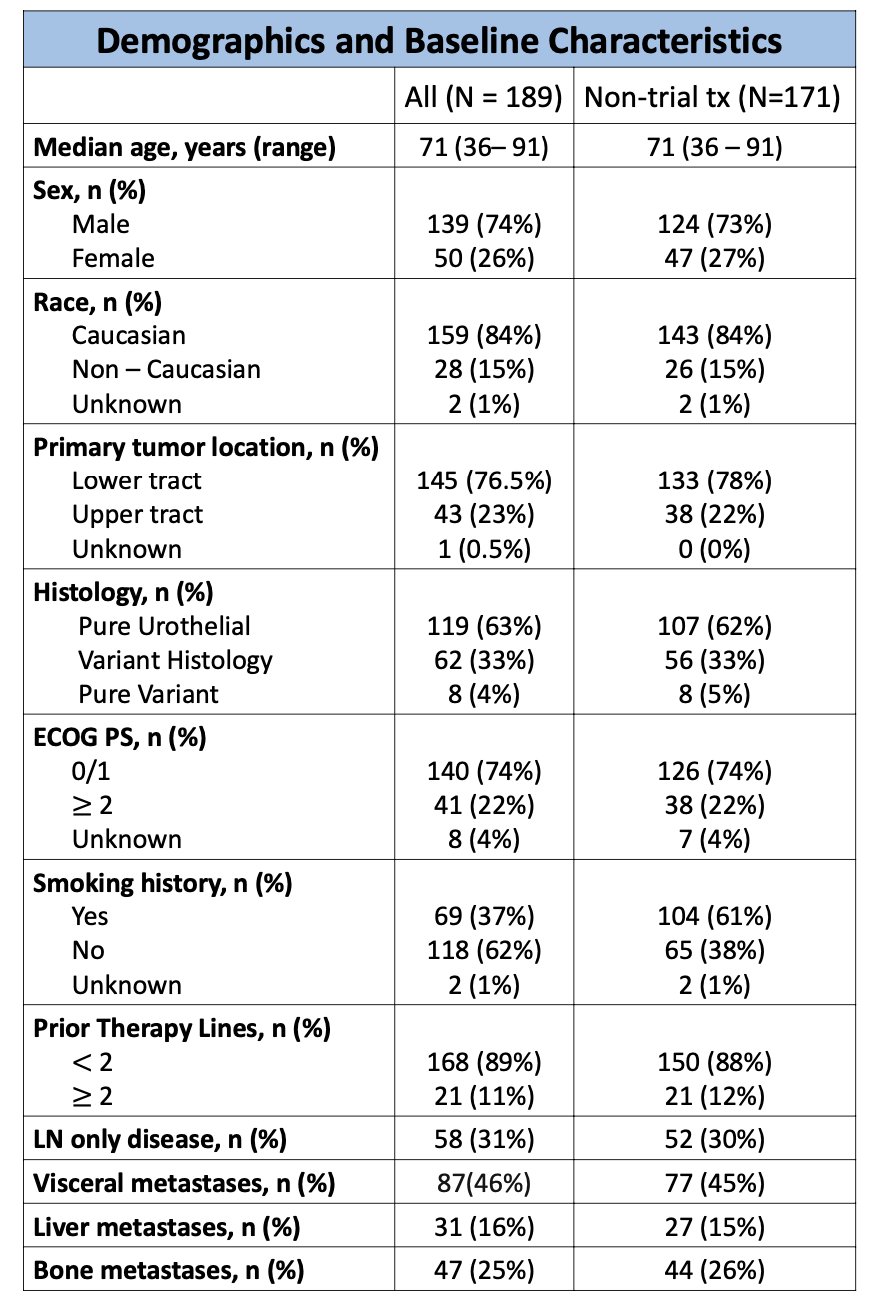
Of the 171 patients treated with Enfortumab vedotin and Pembrolizumab outside of clinical triels, the ORR was 51% and the disease control rate was 84%. With a median follow-up of 5.5 months, the 1-year PFS and OS percentage was 31% and 58%, respectively. The disease control rate appeared to be better in patients who received frontline treatment (88%) versus those who had treatment-refractory disease (80%) or prior ICI exposure (76%).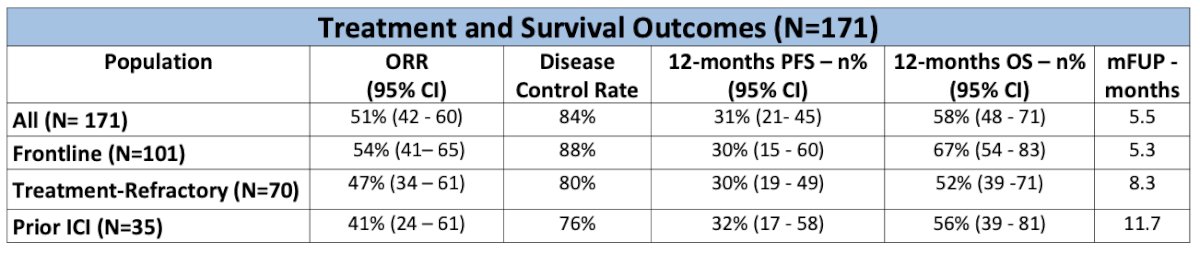
Univariable analysis of clinical characteristics and molecular biomarkers revealed significant associations between TP53 and TERT alterations (versus wild-type) with both progression-free survival (PFS) and overall survival (OS). Additionally, albumin, hemoglobin, neutrophil-to-lymphocyte ratio, and ECOG performance status (ECOG-PS) were significantly associated with OS and PFS in the univariate analysis.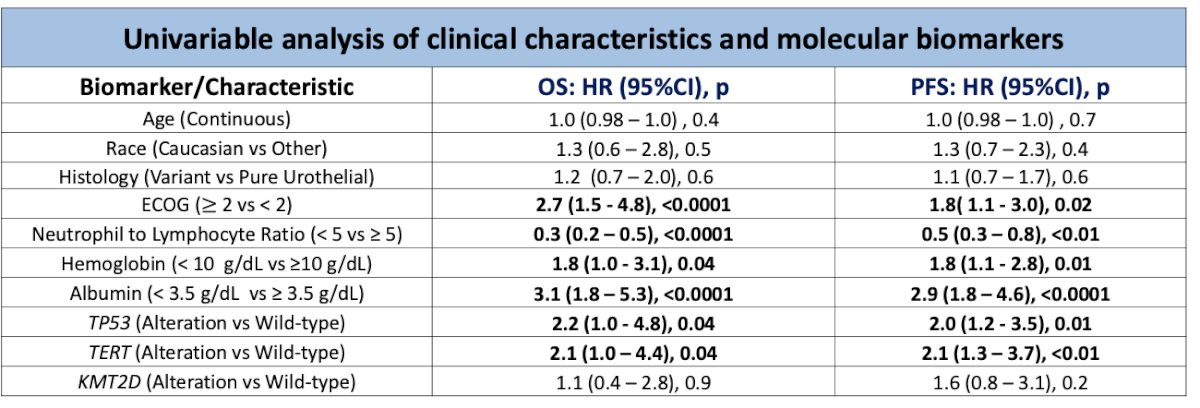
Multivariable analysis adjusting for potential confounders showed that only TP53 remained significantly associated with both OS and PFS. Although KMT2D was not initially associated with any of the time-to-event endpoints, it was found to be significantly associated with PFS after adjusting for clinical confounders.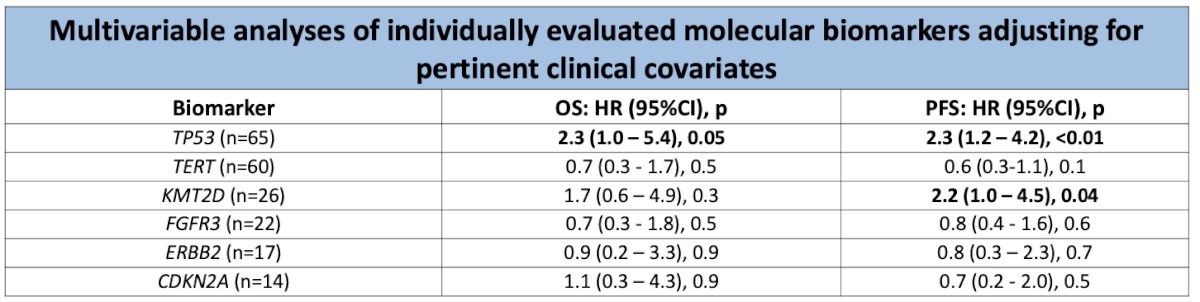
Dr. Jindal wrapped up her poster presentation with the following conclusions:
- Treatment with Enfortumab vedotin and Pembrolizumab outside clinical trials showed robust disease control rate (84%).
- The ORR was more modest in the outside of clinical trials population (51%) compared to frontline clinical trials (68%) EV302.
- Alterations in TP53 and KMT2D may be linked to inferior outcomes (OS and PFS) for patients treated with Enfortumab vedotin and Pembrolizumab.
Presented by: Tanya Jindal, Senior Clinical Research Coordinator, HDF Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:

