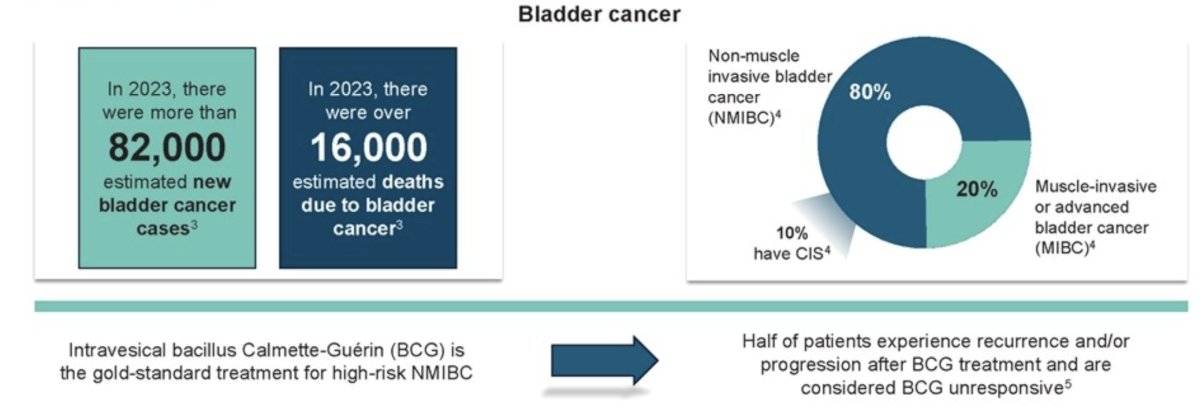
(UroToday.com) The 2024 ESMO annual meeting included a session highlighting urothelial carcinoma trials in progress, with Dr. Joan Palou discussing the trial design of a phase 1/2 study of EG-70 (detalimogene voraplasmid) intravesical monotherapy for patients with BCG-unresponsive NMIBC with CIS. High-risk NMIBC is treated with adjuvant intravesical BCG. However, ∼50% of patients experience recurrence and/or progression after BCG treatment and are considered unresponsive. Bladder sparing therapies for BCG-unresponsive NMIBC address an important and unmet need in this disease space:
EG-70 is an investigational, intravesically administered therapy designed to elicit local stimulation of anti-tumor immune responses in the bladder and drive durable efficacy in patients with BCG-unresponsive NMIBC while mitigating the risk of systemic toxicities from immune stimulation:

Importantly, the nanoparticle formulation can be handled without onerous administration and decontamination procedures for clinicians and patients:

The phase 1 (dose-escalation) portion of the first-in-human LEGEND (NCT04752722) phase 1/2, open-label, multicenter study of EG-70 is complete, and the phase 2 dose was identified. At ESMO 2024, Dr. Palou and colleagues described the ongoing phase 2 portion of the study, which opened to enrollment in May 2023.
Eligibility criteria were as follows:
- Age ≥18 years
- ECOG performance status 0−2
- BCG-unresponsive NMIBC with CIS, with/without resected coexisting papillary tumors, ineligible for, or elected not to undergo, cystectomy
- Satisfactory bladder function with the ability to retain study drug for ≥60 minutes
Patients are treated with EG-70 0.8 mg/mL in 50 mL by intravesical administration on weeks 1, 2, 5, and 6 of a 12-week cycle, for 4 cycles in one of two cohorts:
- BCG-unresponsive (Cohort 1), or
- BCG-naïve or BCG-incompletely treated (Cohort 2)
Patients in either cohort who exhibit stable disease or complete response at week 12 will continue treatment until week 24, whereas patients with progressive disease will discontinue treatment. Patients who experience or maintain complete response at week 24 will receive additional cycles every 12 weeks until week 48, whereas patients with stable disease or progressive disease at week 24 will discontinue treatment. Patients with progressive disease at any time after week 24 will discontinue treatment. Phase 2 primary endpoints include efficacy (complete response rate at week 48) and safety. The trial design for LEGEND is as follows:
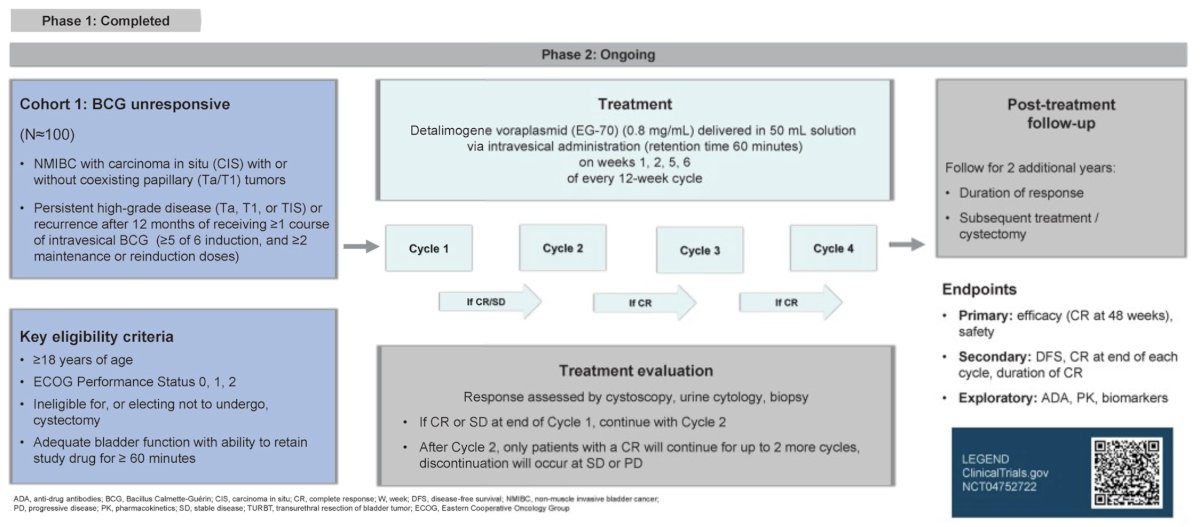
Secondary endpoints include:
- Progression-free survival
- Complete response rate at weeks 12, 24, 36 and 48
- Duration of response
The phase 2 portion of the study will recruit ∼100 patients from sites in North America, Europe, and the Asia-Pacific region.
Presented by: Joan Palou, MD, PhD, FEBU, FRCS (Glas), Fundació Puigvert, Universitat Autonoma de Barcelona, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: LEGEND Trial Investigates EG-70 in Bladder Cancer Patients - Gautier Marcq


