(UroToday.com) The 2024 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Evanguelos Xylinas discussing the association of PD-L1 expression with clinical response to TAR-200 in the phase 2b SunRISe-1 trial. Recurrent non-muscle-invasive bladder cancer (NMIBC) tumors after BCG treatment are characterized by an immunosuppressive environment and increased PD-L1 expression. TAR-200 is a novel gemcitabine intravesical system designed for sustained, local delivery of chemotherapy within the bladder. SunRISe-1 (NCT04640623) is an ongoing randomized, phase 2b study assessing efficacy and safety of TAR-200 + cetrelimab (Cohort 1), TAR-200 alone (Cohort 2), or cetrelimab alone (Cohort 3) in patients with BCG-unresponsive high-risk NMIBC with carcinoma in situ (CIS), with or without papillary disease, who are ineligible for or refusing radical cystectomy. TAR-200 alone is also being assessed in BCG-unresponsive papillary-only high-risk NMIBC (Cohort 4). The investigators previously reported Cohort 2 clinical results showing that TAR-200 monotherapy provides clinically meaningful efficacy and a favorable benefit-risk profile. This study evaluated whether there is an association between PD-L1 expression and response to TAR-200 monotherapy. Additionally, tumor mutational burden and microsatellite instability were also measured.
Tumor tissue was collected during screening by transurethral resection of bladder tumor or bladder biopsy from Cohort 2 in patients enrolled in SunRISe-1. The SunRISe-1 cohort study design and biomarker measurement is as follows:
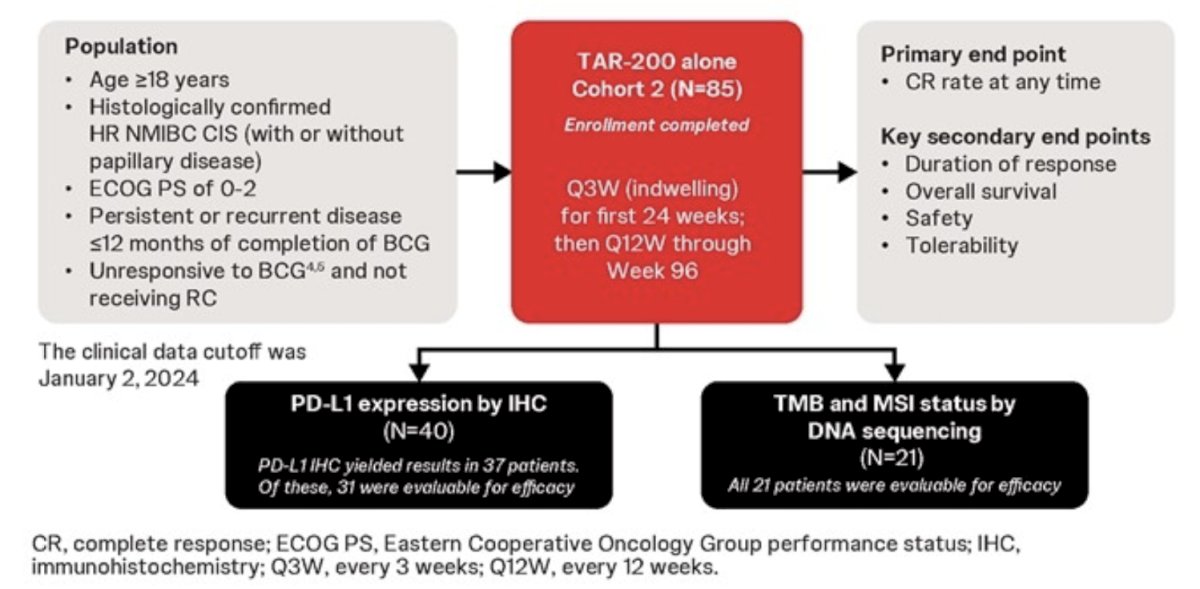
PD-L1 was measured by immunohistochemistry (22C3) and scored for combined positive scores (CPS) with cutoffs of 1 and 10. DNA isolation and sequencing using the Illumina TSO-500 panel measured tumor mutational burden and microsatellite instability scores. Clinical outcomes in these analyses used the January 2, 2024 clinical data cutoff.
As of the data cutoff, 85 patients had received TAR-200 monotherapy:
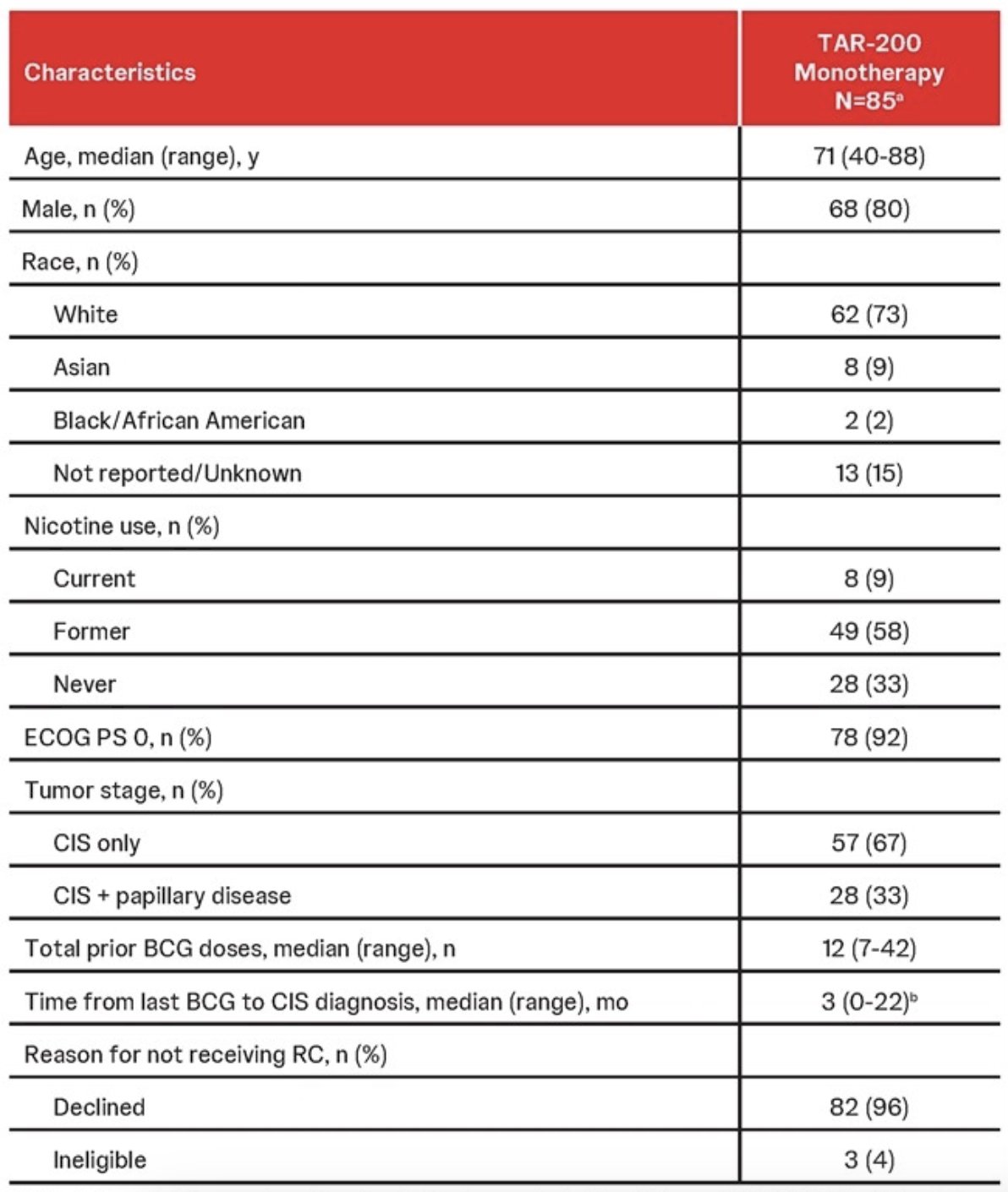
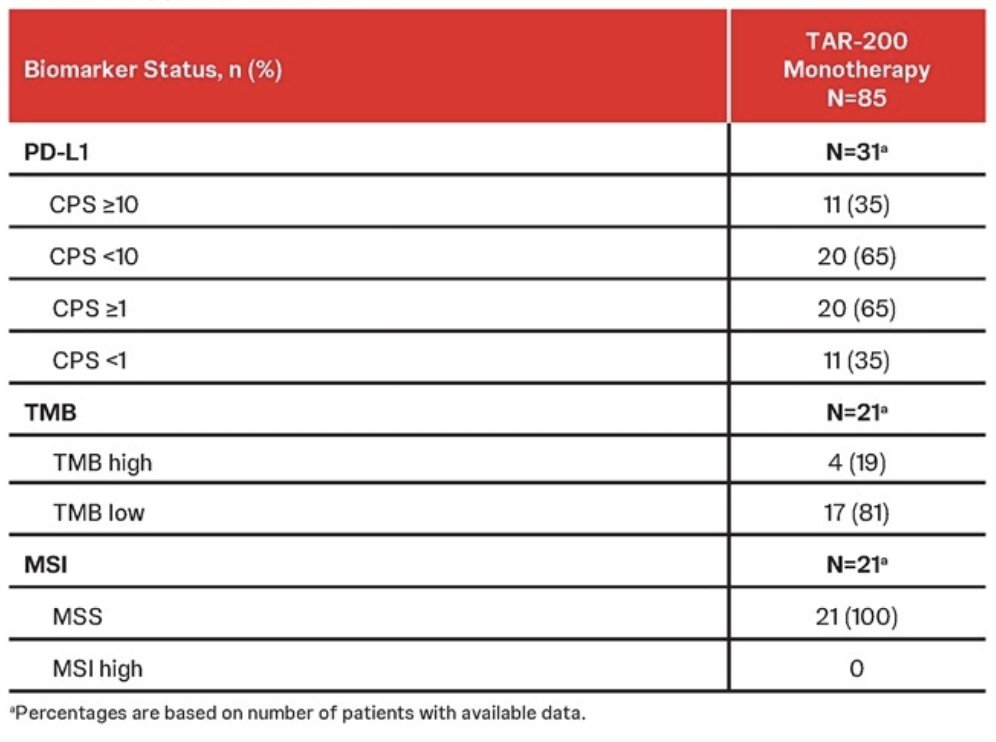
PD-L1 expression was measured in a subset of 40 patients in Cohort 2 with available tumors, with 31 yielding PD-L1 results. Of these, 11 (35%) tumors had PD-L1 CPS measurements ≥10, and 20 (65%) had CPS scores ≥1. Tumor mutational burden and MSI were assessed in 21 patients using baseline DNA isolation and sequencing available tumor tissue. There were 17 patients (81%) TMB low and 4 (19%) TMB high. All 21 patients had MSS tumors (100%), with a median 1.6% of unable microsatellite sites and no MSI high tumors:
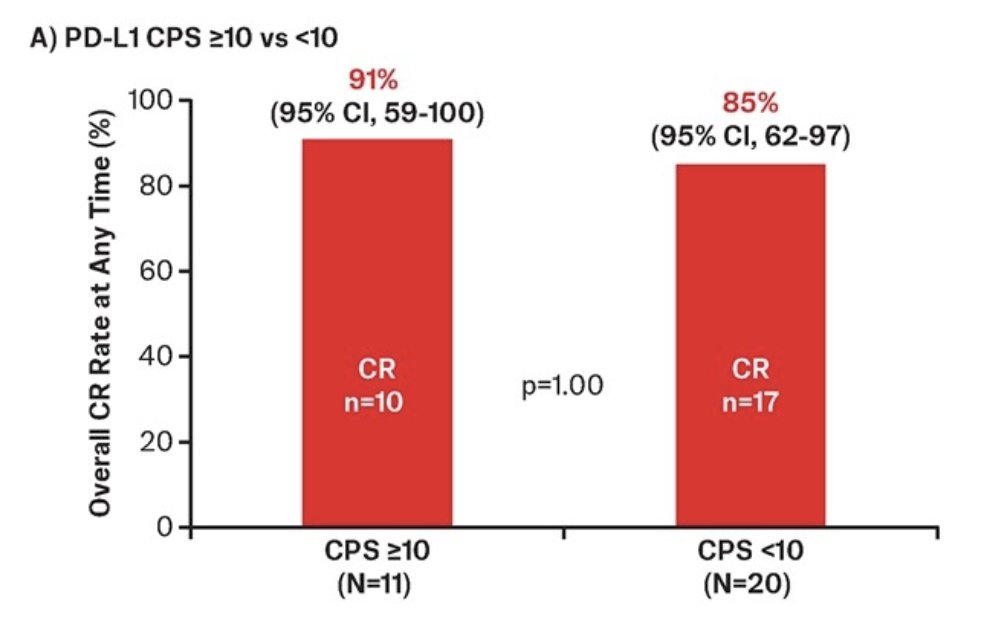
At the data cutoff, 58 patients were efficacy evaluable, with TAR-200 monotherapy showing a complete response rate at any time of 83% (95% CI 71-91). Among 31 patients with PD-L1 data that were evaluable for clinical efficacy, the complete response rate was 87% (27/31). Complete response rates of 91% and 85% were observed in the CPS ≥10 and CPS <10 subgroups, respectively:
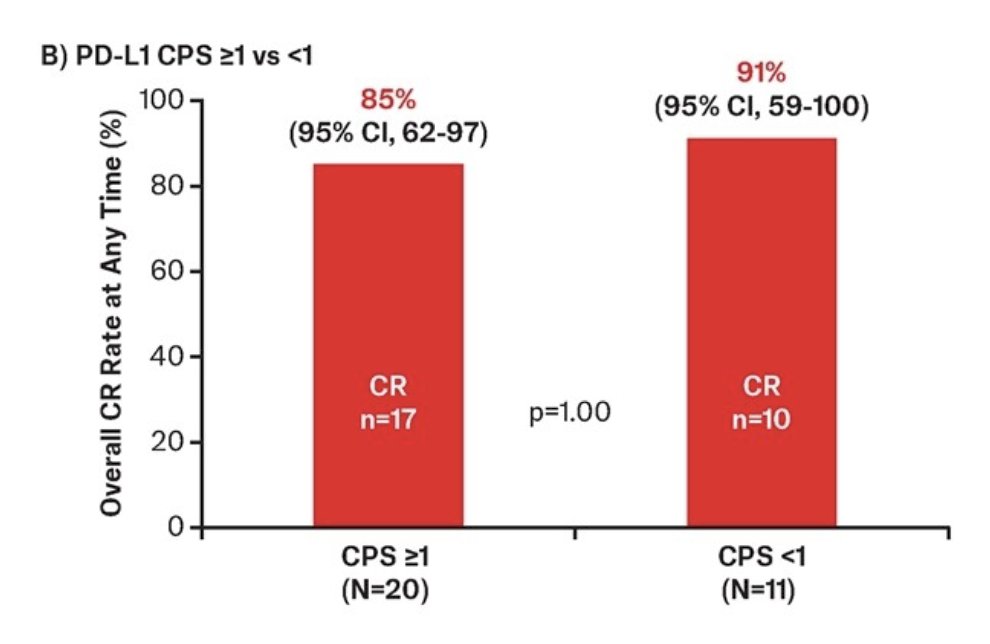
Complete response rates of 85% and 91% were observed in the CPS ≥1 and CPS <1 subgroups, respectively:
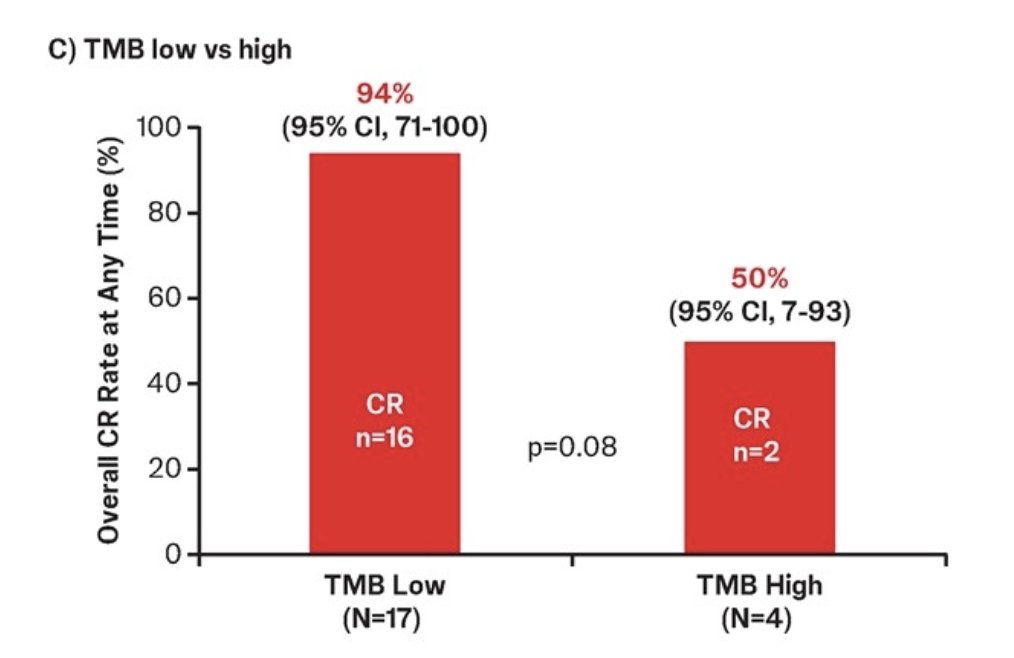
In 21 patients with available tumor mutational burden data who were treated with TAR-200 monotherapy and were efficacy evaluable, the complete response rate was 86%. Complete response rates of 94% and 50% were observed in the TMB low and TMB high subgroups, respectively:
In 21 patients with available MSI scores who were treated with TAR-200 monotherapy and were efficacy evaluable, the complete response rate was 86% (18/21). Across biomarker subgroups analyzed, no statistically significant differences in complete response rates were observed.
Dr. Xylinas concluded his presentation by discussing the association of PD-L1 expression with clinical response to TAR-200 in the phase 2b SunRISe-1 trial with the following take-home points:
- Across subgroups of patients with BCG unresponsive high risk NMIBC with CIS based on PD-L1 status, TAR-200 monotherapy showed a high complete response rate (85-91%)
- A high complete response rate (94%) was observed in the subgroup of patients with TMB low status who received TAR-200 monotherapy; the sample size in the TMB high subgroup was very small (n = 4)
- All patients with available MSI scores had MSS tumors; the complete response rate among patients with MSS tumors was also high (86%)
Presented by: Evanguelos Xylinas, MD, PhD, Bichat Claude Bernard Hospital, Public Assistance of Paris Hospitals, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


