(UroToday.com) The 2024 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Alexandra Drakaki, discussing ctDNA clearance with neoadjuvant durvalumab + tremelimumab + enfortumab vedotin for cisplatin-ineligible muscle invasive bladder cancer from the safety run-in cohort of the phase 3 VOLGA trial. The VOLGA safety run-in of neoadjuvant durvalumab + tremelimumab + enfortumab vedotin in cisplatin-ineligible patients with muscle invasive bladder cancer has previously shown promising results (NCT04960709). Pathologic complete response was seen in 6/17 patients and pathologic non-muscle invasive response was seen in 9/17 patients. The safety profile was manageable, with the most common treatment related adverse events being fatigue, maculopapular rash, dysgeusia, dry mouth, and pruritus. Emerging data also suggests that ctDNA may help predict treatment benefit in patients with muscle invasive bladder cancer. At ESMO 2024, Dr. Drakaki and colleagues presented an exploratory analysis of plasma ctDNA from the safety run-in.
Eligible patients were ≥18 years with cisplatin-ineligible MIBC (clinical stage T2-4aN0-N1M0/T1N1M0). Patients received 3 cycles of neoadjuvant therapy every 3 weeks: durvalumab (1500 mg; day 1 each cycle) + tremelimumab (75 mg; day 1 cycle 1, and day 8 cycle 2) + enfortumab vedotin (1.25 mg/kg; days 1 and 8 each cycle), followed by radical cystectomy, then 9 cycles of adjuvant durvalumab (every 4 weeks; day 1 each cycle) and tremelimumab (day 1, cycle 1 only). The trial design for VOLGA is as follows:
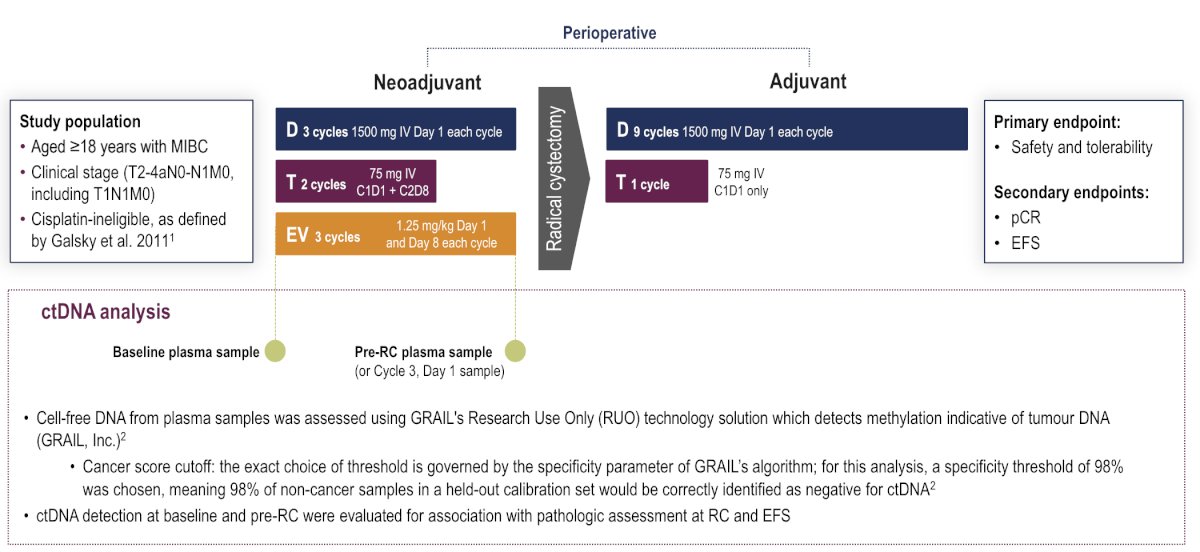
Cell-free DNA from plasma samples was assessed using GRAIL’s cancer research solution, which detects methylation indicative of ctDNA. ctDNA detection at baseline (C1D1) and on/after neoadjuvant treatment prior to radical cystectomy (C3D1 and pre-radical cystectomy time points) was evaluated for association with pathologic response at radical cystectomy and event-free survival:
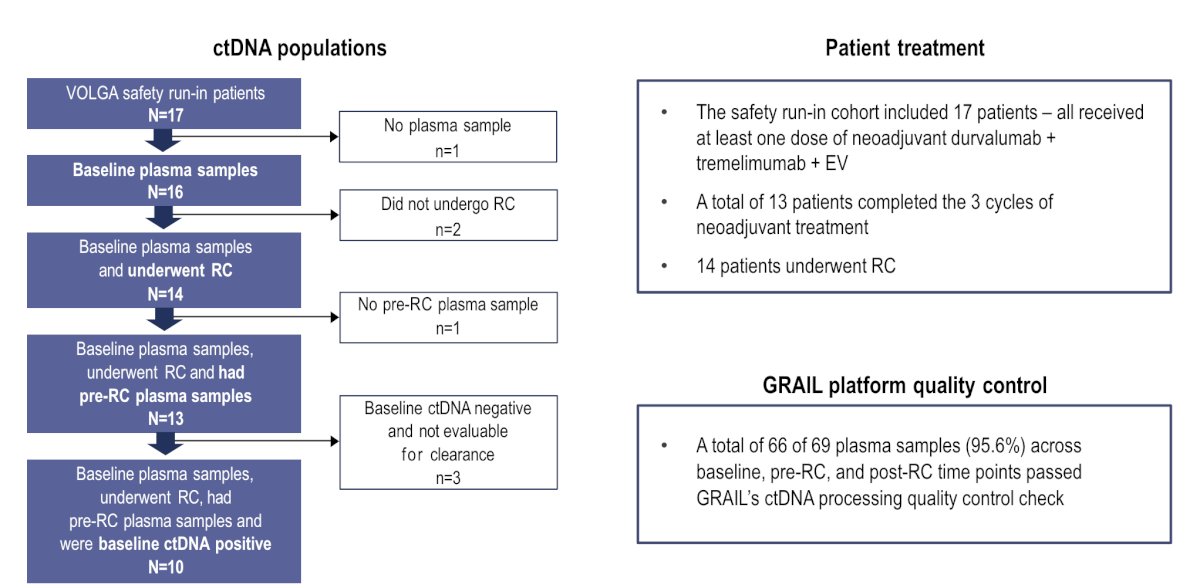
Among 69 plasma samples across baseline, pre-radical cystectomy, and post-radical cystectomy time points, 66 (96%) passed a ctDNA processing quality control check. Of the 17 patients in the safety run-in cohort, 16 had baseline plasma samples with a ctDNA-positive rate of 62.5% (10/16 patients):
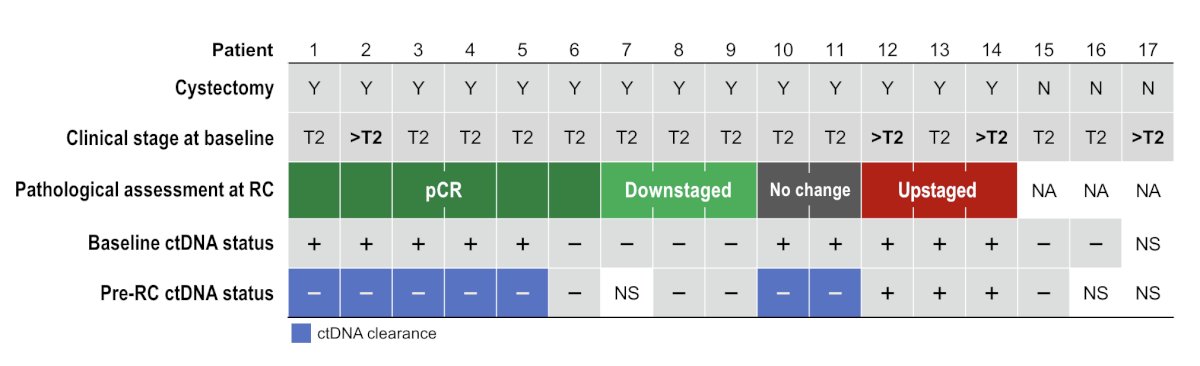
Among these patients, 13 underwent radical cystectomy and had a pre-radical cystectomy sample. The ctDNA-positive rate at C3D1/pre-radical cystectomy was 23% (3/13 patients), and 7 patients had ctDNA clearance, which was associated with pathologic response. Among 6 patients with pathologic complete response, ctDNA clearance was observed in 5 patients, and among 3 downstaged patients, ctDNA was negative at both time points:

Event free survival was assessed in 13 patients who completed radical cystectomy, of which 10 were ctDNA positive at baseline, and 3 were ctDNA negative at baseline. Longer event free survival was observed in the ctDNA clearance and ctDNA negative groups compared with the ctDNA positive group:

Dr. Drakaki concluded her presentation by discussing ctDNA clearance from the the phase 3 VOLGA trial with the following take-home points:
- In a small safety run-in cohort of the VOLGA study, clearance of plasma ctDNA during the neoadjuvant phase was associated with a favorable outcome in cisplatin ineligible patients with muscle invasive bladder cancer treated with durvalumab + tremelimumab + enfortumab vedotin
- This exploratory analysis supports ctDNA as a potential biomarker and may help predict treatment benefits in patients with muscle invasive bladder cancer. There is continued collection of samples for ctDNA analysis in the main VOLGA trial
- The GRAIL RUO technology solution had a high quality-control pass rate for ctDNA assessment of plasma samples from patients with muscle invasive bladder cancer
Presented by: Alexandra Drakaki, MD, UCLA, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


