(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Mini oral session: GU tumours, non-prostate. Dr. Matthew D. Galsky presented the preliminary efficacy and safety results of Disitamab Vedotin (DV) With Pembrolizumab (P) for the treatment-naive HER2-Expressing, locally advanced or metastatic urothelial carcinoma.
Dr. Galsky began by discussing Vedotin-based antibody-drug conjugates (ADCs), which have transformed the treatment landscape for previously untreated locally advanced/metastatic urothelial carcinoma (la/mUC). Novel biomarkers may help inform treatment strategies and potentially improve outcomes further. Data confirm that approximately 60% to 80% of patients with urothelial carcinoma demonstrate HER2 expression.1
Disitamab vedotin (DV; RC48-ADC) is an investigational ADC comprising a fully humanized HER2-directed monoclonal antibody, disitamab, conjugated to the microtubule-disrupting agent Monomethyl auristatin E (MMAE) via protease-cleavable mc-vc linkers. Similar to other vedotin-based ADCs, DV can mediate immunogenic cell death following the release of MMAE within target tumor cells, providing the rationale to combine DV with immune checkpoint inhibitors (ICIs) such as Pembrolizumab (Anti-PD-1).2
Data from combinations of DV with PD-1 inhibitors, such as Toripalimab, have previously demonstrated promising antitumor activity with a manageable safety profile in patients with la/mUC, as presented by Dr. Sheng at this ESMO meeting.3 Dr. Galsky reported the safety and preliminary efficacy results of the safety run-in (Part 1) of Cohort C (DV + Pembrolizumab) in the open-label, phase 2, multicenter study RC48G001 (NCT04879329) in treatment-naive patients with HER2-expressing (HER2-positive: IHC 3+, or IHC 2+ and ISH-positive or HER2-low: IHC 2+ and ISH-negative, or IHC 1+), the study design is outlined below:
The Cohort C included 20 patients, most of whom were male (75%). The median age was 75 years. In terms of HER2 status, 30% had HER2-positive status, while 70% had HER2-negative status. The primary tumor was located in the bladder for 60% of the patients, and the majority had metastatic visceral disease (75%). The median follow-up for this cohort was 9 months.
The confirmed overall response rate (ORR) was 75% (95% CI 50.9-91.3), with 7 patients (35%) achieving a complete response and 8 patients (40%) achieving a partial response. Patients in both the HER2-positive and HER2-low groups responded to the DV+P treatment. In the HER2-positive group, the ORR was 66.7%, while in the HER2-low group, it was 78.6%. The median duration of response was not reached.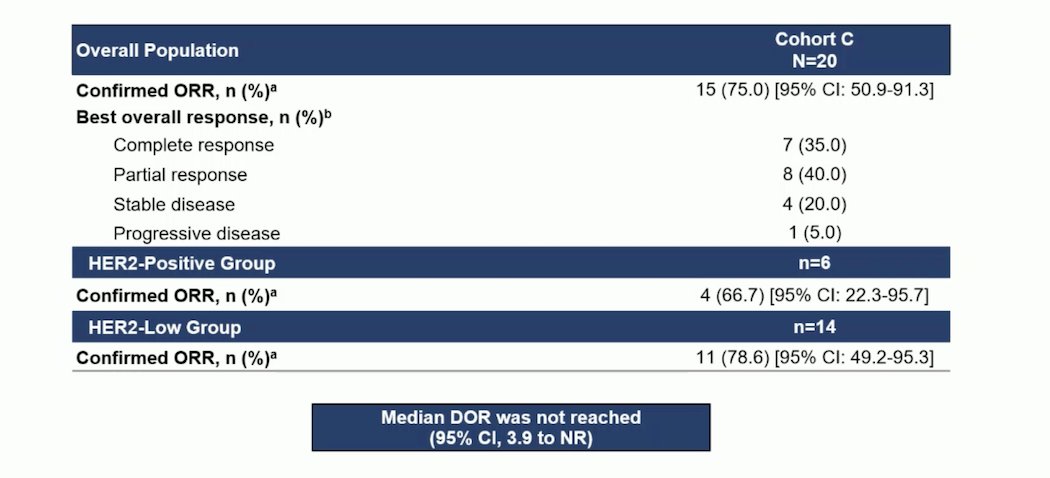
The percent change in the sum of diameters from baseline is illustrated in the spider plot below, again confirming response in both the HER2-positive and HER2-low groups.
In terms of safety, there were no grade 5 treatment-related adverse events (TRAEs). The most common (≥10%) treatment-emergent immune-mediated adverse events (AEs) were diarrhea (10%) and maculopapular rash (10%). Notably, no cases of pneumonitis or interstitial lung disease were observed.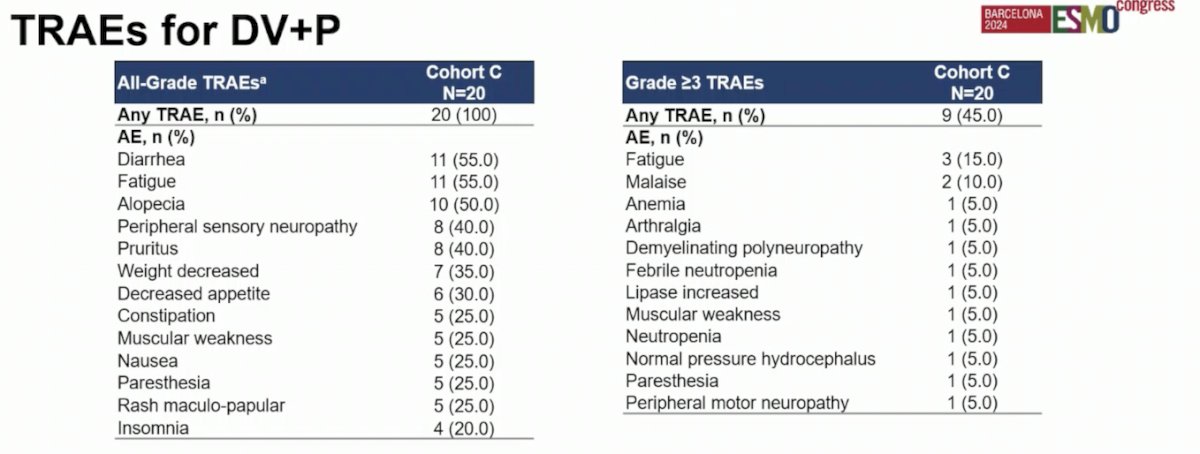
A total of 19 patients (95%) required a dose modification of DV, and 80% required a dose modification of Pembrolizumab. The most common treatment-emergent adverse events (TEAEs) leading to dose delays for DV (≥10%) were peripheral sensory neuropathy, decreased appetite, fatigue, and paresthesia. For Pembrolizumab (≥5%), the most common TEAEs leading to dose delays were anemia, decreased appetite, and fatigue.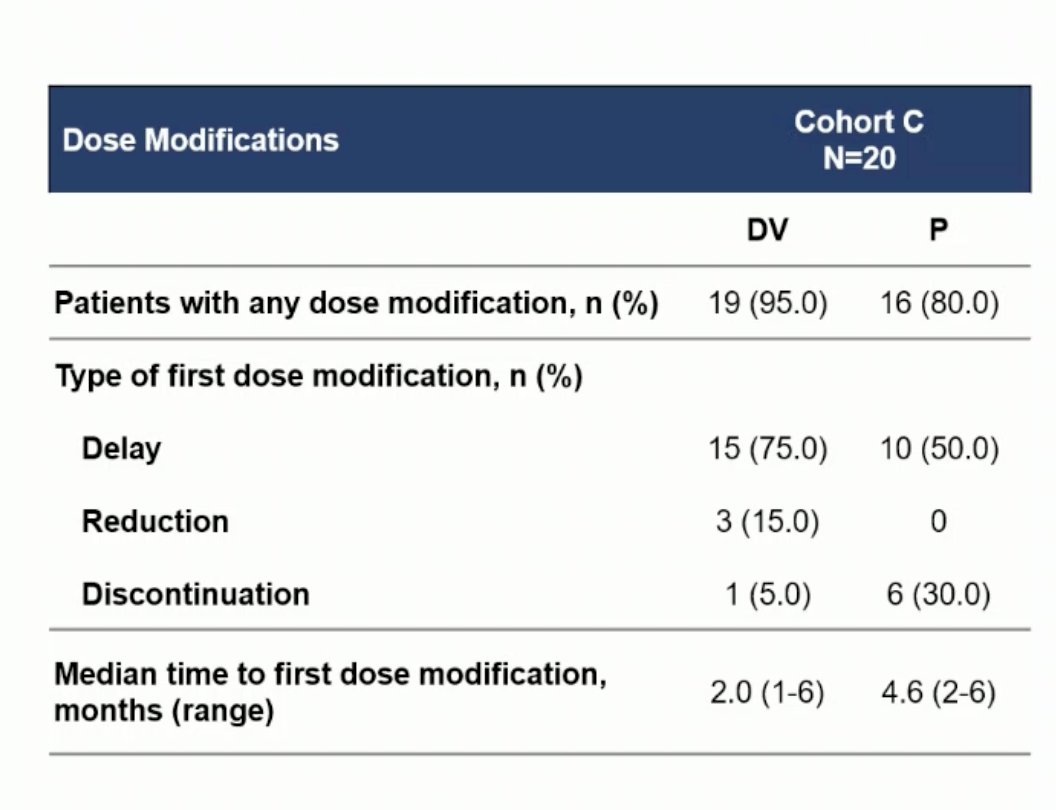
Dr. Galsky concluded his presentation with the following take-home messages:
- DV+P demonstrated encouraging preliminary activity in treatment-naive patients with HER2-expressing la/mUC (n=20, Cohort C).
- Responses to DV+P were observed in both patients with HER2-positive and HER2-low la/mUC.
- These results with DV+P are consistent with updated data from the phase 1b/2 study of DV plus the PD-1 inhibitor toripalimab.
- The phase 3 study (SGNDV-001; NCT05911295) is currently exploring the efficacy and safety of first-line DV+P compared with chemotherapy. Enrollment is ongoing/planned in North America, Latin America, Europe, Australia, and Asia.
Presented by: Matthew D. Galsky, MD, FASCO, Professor of Medicine, Icahn School of Medicine at Mount Sinai, Director, Genitourinary Medical Oncology, Associate Director, Translational Research, Tisch Cancer Institute, New York, NY
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related content: Disitamab Vedotin: Promising HER2-Targeted Therapy for Urothelial Cancer - Matthew Galsky
ESMO 2024: Invited Discussant: Futibatinib Plus Pembrolizumab, Retrospective Assessment of Nectin-4 Expression, and Disitamab Vedotin
- Uzunparmak B, Haymaker C, Raso G, Masciari S, Wang L, Lin H, Gorur A, Kirby B, Cimo AM, Kennon A, Ding Q, Urschel G, Yuan Y, Feng G, Rizvi Y, Hussain A, Zhu C, Kim P, Abbadessa G, Subbiah V, Yap TA, Rodon J, Piha-Paul SA, Meric-Bernstam F, Dumbrava EE. HER2-low expression in patients with advanced or metastatic solid tumors. Ann Oncol. 2023 Nov;34(11):1035-1046. doi: 10.1016/j.annonc.2023.08.005. Epub 2023 Aug 22. PMID: 37619847.
- Li L, Xu MZ, Wang L, Jiang J, Dong LH, Chen F, Dong K, Song HF. Conjugating MMAE to a novel anti-HER2 antibody for selective targeted delivery. Eur Rev Med Pharmacol Sci. 2020 Dec;24(24):12929-12937. doi: 10.26355/eurrev_202012_24196. PMID: 33378043.
- Sheng X, Wang L, He Z, Shi Y, Luo H, Han W, Yao X, Shi B, Liu J, Hu C, Liu Z, Guo H, Yu G, Ji Z, Ying J, Ling Y, Yu S, Hu Y, Guo J, Fang J, Zhou A, Guo J. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J Clin Oncol. 2024 Apr 20;42(12):1391-1402. doi: 10.1200/JCO.22.02912. Epub 2023 Nov 21. PMID: 37988648; PMCID: PMC11095880.


