(UroToday.com) The 2024 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Michiel Van der Heijden, discussing updated results from SunRISe-1 assessing TAR-200 +/- cetrelimab and cetrelimab alone in patients with BCG-unresponsive high-risk NMIBC. Unfortunately, patients with BCG unresponsive high-risk NMIBC have limited treatment options. Standard of care for these patients is radical cystectomy, which is a life-changing operation associated with considerable morbidity and impact on quality of life, and a 90 day mortality risk of up to 8%. Limited US FDA approved treatment options are available to treat BCG-unresponsive high-risk NMIBC CIS, with 12 month complete response rates of:
- 19% with pembrolizumab1
- 23% with nadofaragene firadenovec2
- 45% with nogapendekin alfa inbakicept + BCG3
Cetrelimab is an anti-PD-1 agent with an efficacy and safe profile consistent with approved anti-PD-1 agents.
TAR-200 is an intravesical targeted releasing system designed to provide sustained delivery of gemcitabine in the bladder over 3 weeks. SunRISe-1 (NCT04640623) is an ongoing randomized, phase 2b study evaluating efficacy and safety of TAR-200 + cetrelimab (Cohort 1), TAR-200 alone (Cohort 2), or cetrelimab alone (Cohort 3) in patients with BCG unresponsive high-risk NMIBC ineligible for/refusing radical cystectomy. TAR-200 alone is also being assessed in patients with papillary disease only (Cohort 4). At ESMO 2024, Dr. Van der Heijden reported results from Cohort 1-3.
Eligible patients (≥18 years) had histologically confirmed carcinoma in situ (CIS) ± papillary disease (high-grade Ta, any T1) with last dose of adequate BCG ≤12 months of CIS diagnosis, and ECOG performance status 0-2. TAR-200 was dosed Q3W to week 24 then Q12W to week 96, while cetrelimab was dosed Q3W to week 78. The primary end point was complete response rate at any time, and secondary end points included duration of response, overall survival, safety and tolerability. The SunRISe-1 trial design is highlighted below:
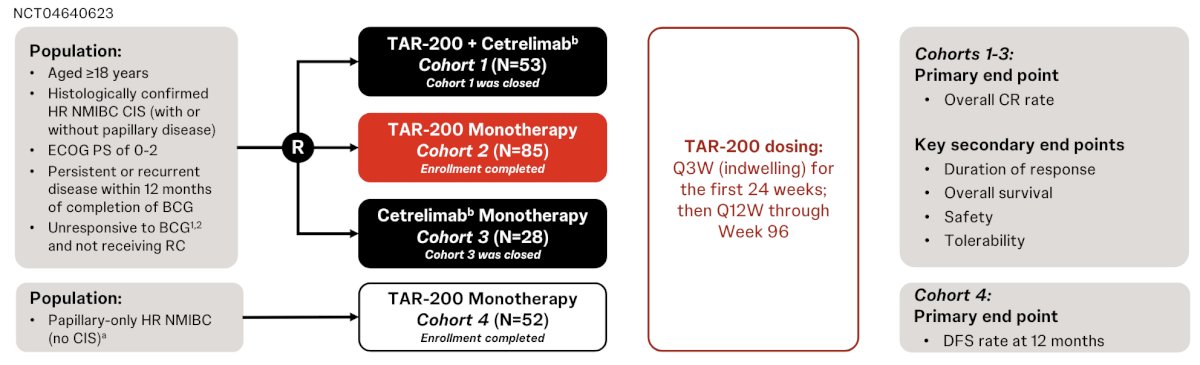
Assessments included local cystoscopy, centrally assessed urine cytology Q12W, and centrally assessed biopsy at weeks 24 and 48.
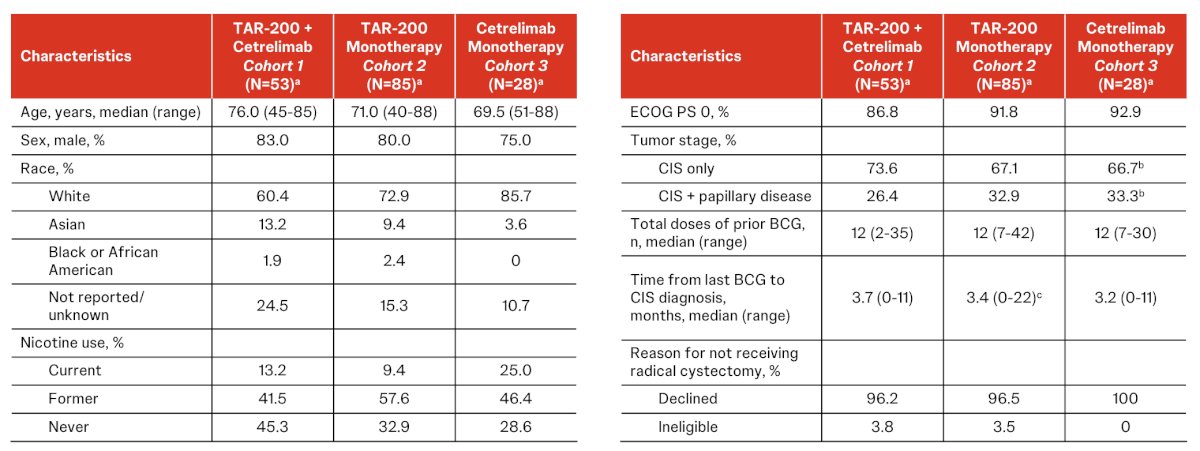
At a May 13, 2024 data cutoff, 53 patients in Cohort 1, 85 in Cohort 2, and 28 in Cohort 3 were treated (median age 71.5 years, 80% male, 31% papillary disease). The baseline characteristics across the cohorts is as follows:
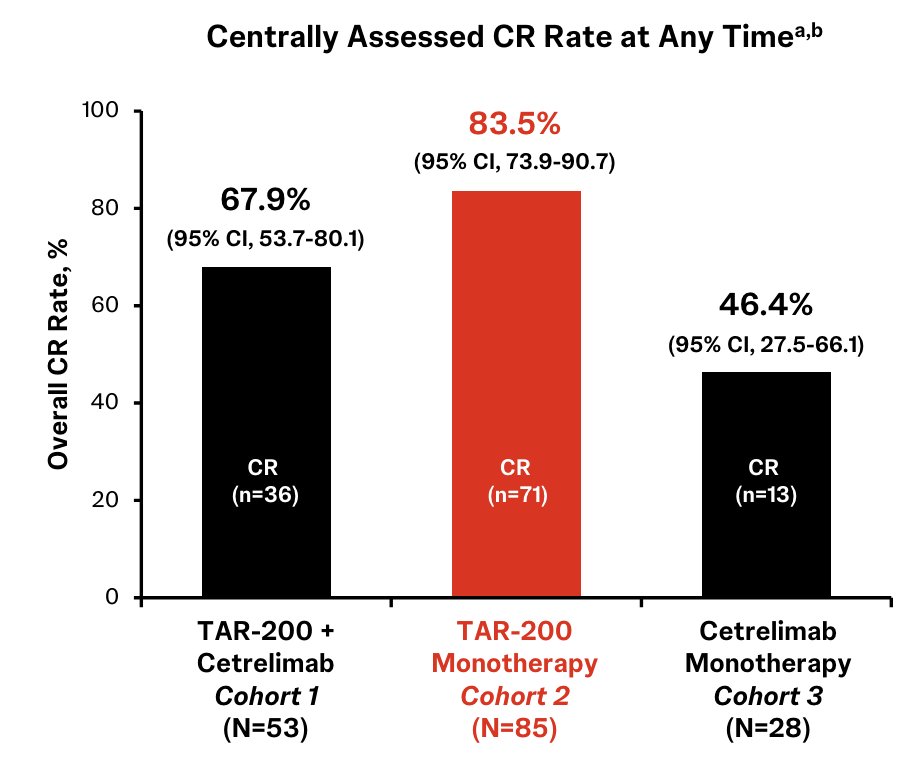
Centrally confirmed complete response rates in Cohort 1, Cohort 2, and Cohort 3 were 68%, 84%, and 46%, respectively:
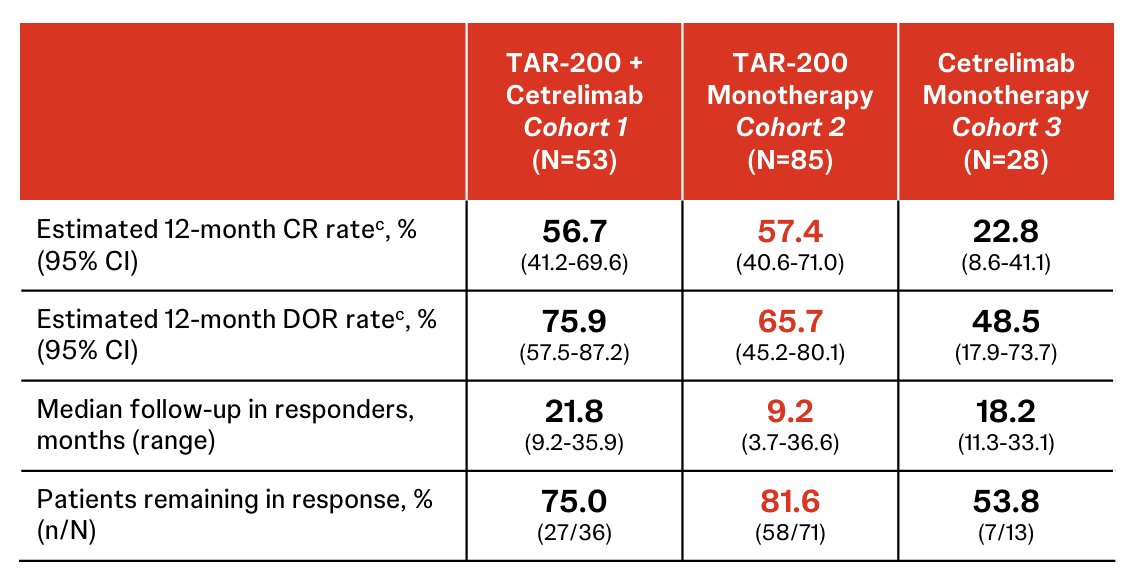
In Cohort 2, the estimated 12-month complete response rate was 57.4%, estimated 12-month duration of response rate was 65.7%, median follow-up in responders was 9.2 months (range: 3.7-36.6), and patients remaining in response was 81.6%:
Overall, most adverse events were grade 1 or 2, with higher rates of grade >= 3 treatment related adverse events were observed with the combination regimen (35.8%), than with TAR-200 (9.4%) or cetrelimab (7.1%) monotherapy. Low discontinuation rates due to treatment-related adverse events were seen with TAR-200 (Cohort 2, 6%) and cetrelimab (Cohort 3, 7%) alone, with higher rates in the combination (Cohort 1, TAR-200 26% or cetrelimab 23%).
Dr. Van der Heijden concluded his presentation discussing updated results from SunRISe-1 with the following take-home points:
- TAR-200 monotherapy provides the highest single agent complete response rate of 84% in patients with BCG-unresponsive high-risk NMIBC, based on published data, without the need for re-induction
- Responses to TAR-200 monotherapy are highly durable; 82% of patients remain in response after a median follow-up of 9.2 months
- TAR-200 monotherapy was well tolerated, with few grade >= 3 treatment related adverse events or treatment related adverse events leading to discontinuation
- Cetrelimab monotherapy provided a complete response rate comparable to other anti-PD-(L)1 agents
- SunRISe-1 results indicate a more favorable risk benefit profile for TAR-200 monotherapy compared with TAR-200 + cetrelimab or cetrelimab monotherapy in BCG-unresponsive high risk NMIBC
- Results from SunRISe-1 Cohorts 1-3 support the prioritized development of TAR-200 monotherapy in patients with BCG-unresponsive high-risk NMIBC
Presented by: Michiel S. Van der Heijden, MD, PhD, Netherlands Cancer Institute, Amsterdam, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021 Jan;22(1):107-117.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1)
Related Content: New Data from TAR-200 Phase 2b SunRISe-1 Study Show 84 Percent Complete Response Rate in Patients with High-Risk Non-Muscle-Invasive Bladder Cancer


