(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Presidential Symposium II: Practice-changing trials. Dr. Thomas B. Powles presented the results of the NIAGARA trial, the first randomized phase 3 trial of neoadjuvant durvalumab plus chemotherapy followed by radical cystectomy and adjuvant durvalumab in muscle-invasive bladder cancer.
Dr. Powles began by highlighting that NIAGARA is the first phase 3 trial testing perioperative immune checkpoint inhibitor (Durvalumab) combined with NAC in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). Additionally, it is the largest phase 3 trial that has ever been done in bladder cancer and, as we would see, it is poised to be practice-changing in this space of bladder cancer.
Neoadjuvant cisplatin-based chemotherapy (NAC) with radical cystectomy (RC) improves overall survival (OS) versus RC alone and has been the standard of care treatment for muscle-invasive bladder cancer (MIBC) for the past 40 years. However, approximately 50% of patients will relapse within three years and eventually die of the disease.1
In the setting of MIBC, immune checkpoint inhibitors as adjuvant monotherapy have demonstrated improved disease-free survival in Phase 3 studies for patients at high risk of recurrence after surgery, as seen in the CheckMate 274 (Nivolumab) and AMBASSADOR Alliance A031501 trial (Pembrolizumab).2,3
The investigators hypothesized that perioperative immune checkpoint inhibitors could improve long-term clinical outcomes by priming anti-tumor immunity before surgery and eradicating micrometastatic disease after surgery. Perioperative durvalumab was shown to be safe and efficacious in a Phase II Trial SAKK 06/17.4
The NIAGARA trial enrolled adult patients with cisplatin-eligible MIBC (cT2-TaN0/1M0), including those with divergent differentiation or histologic subtypes. All participants were evaluated and confirmed as suitable for RC, with the cisplatin eligibility adjusted to a CrCl of ≥40 mL/min. In the Durvalumab arm, patients were randomized to receive Durvalumab 1500 mg IV Q3W with Gemcitabine + Cisplatin (GC) for 4 cycles, followed by RC and adjuvant Durvalumab 1500 mg IV Q4W for up to 8 cycles. In the comparator arm, patients were randomized to receive GC followed by RC and did not receive any adjuvant treatment.
The dual primary endpoints were:
- Event-free survival (EFS), defined as progressive disease that precluded RC, recurrence after RC, failure to undergo RC by the expected surgery date, or death from any cause.
- Pathological complete response rate (pCR), evaluated by blinded central pathology review (BCPR).
The study design is shown below:
The statistical analysis plan included a multiple testing procedure with an alpha-exhaustive recycling strategy and a gatekeeping strategy. This approach was applied to the dual primary endpoints (EFS and pCR) and then to the secondary endpoints of OS and 5-year OS. The 5% alpha (2-sided) was allocated between the two primary endpoints: 0.1% for pCR and 4.9% for EFS, with the remaining alpha reserved for the OS analysis, as illustrated in the graphic below:
The investigators planned one pCR analysis approximately 6 months after the last patient was randomized in the intention-to-treat (ITT) population. For this interim analysis, the estimated number of events was 410 for EFS and 288 for OS in the ITT population, and 433 for EFS and 305 for OS in the overall population.
A total of 1,530 patients were enrolled in the NIAGARA trial, and 1,063 were eventually randomized: 533 to the Durvalumab arm and 530 to the comparator arm in the intention-to-treat (ITT) population. The median time from the last dose of neoadjuvant therapy to RC was 39 days for the Durvalumab arm and 38 days for the comparator arm. At the time of data cutoff, no patients were still receiving study treatment.
The patient disposition and baseline characteristics were well-balanced, as expected in a randomized trial with over 1,000 patients. Notably, 14% of patients in the Durvalumab arm and 17% in the comparator arm had UC with divergent differentiation or histologic subtypes. Additionally, 5% of patients in the Durvalumab arm and 6% in the comparator arm had N1 disease at baseline.
Dr Powles mentioned that 85% of randomized patients underwent successfully a RC and 70% received adjuvant durvalumab.
EFS, as assessed by blinded independent central review (BICR) in the intention-to-treat (ITT) population, showed a significant reduction in the hazard of events in the Durvalumab arm. The EFS at 2 years was 67.8% in the Durvalumab arm compared to 59.8% in the comparator arm. The hazard ratio (HR) for EFS was 0.68 (95% CI: 0.56–0.82, p<0.0001).
A sensitivity analysis of EFS, which censored patients who did not undergo RC and accounted for the imbalance in RC between the two arms, showed almost identical results. The EFS rate at 24 months was 73.5% in the Durvalumab arm and 67.9% in the comparator arm. The hazard ratio was 0.69 (95% CI: 0.56–0.86).
In the subgroup analysis of EFS, Durvalumab was favored across almost all subgroups, except for the N1 disease at baseline group, where the confidence interval crossed 1 (HR 0.75, 95% CI: 0.22–1.64), which could be attributed to the small sample size in this group (n=58).
The second primary endpoint, pCR, was reported in two separate analyses: a formal analysis in January 2022 and a re-analysis in April 2024. The planned formal analysis for pCR was not statistically significant (p=0.0038) as the threshold for significance was a p-value of 0.001. However, this analysis incorrectly classified 59 evaluable samples as non-responders rather than their true result. The re-analysis, which included these 59 samples and identified 28 additional pCRs, showed nominal statistical significance in favor of the Durvalumab arm (p=0.0005).

Secondary endpoint: Overall survival
The median follow-up for the OS analysis in censored patients was 46.3 months (range 0.03–64.7). At 24 months, 82.2% of patients in the Durvalumab arm were alive, compared to 75.2% in the comparator arm. The hazard ratio for OS was 0.75 (95% CI: 0.59–0.93, p=0.016).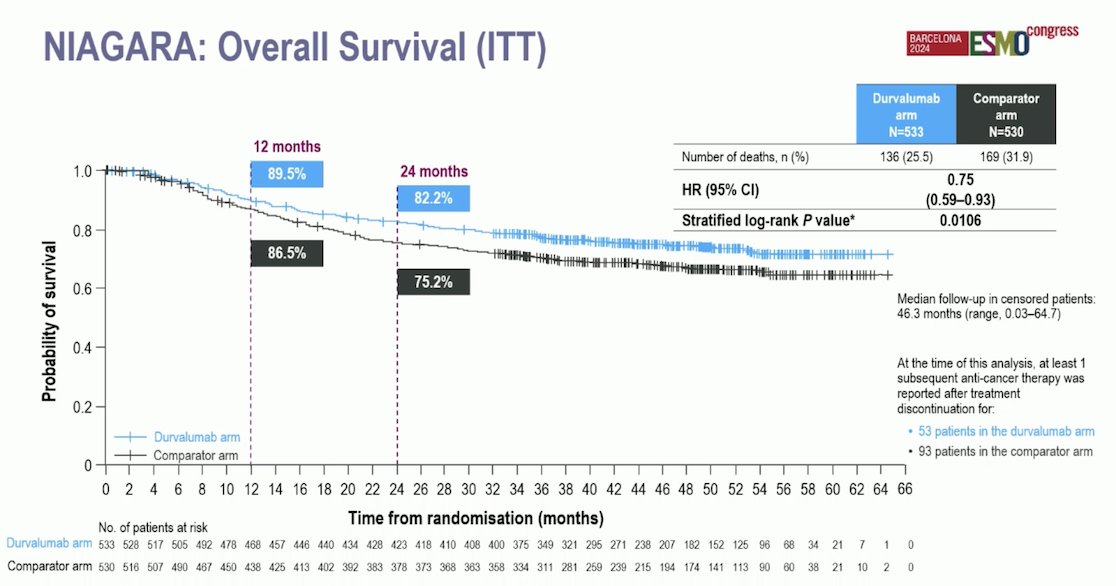
The subgroup analysis of OS was consistent. Durvalumab significantly increased survival in almost all subgroups. The forest plot for this analysis is shown below. 
In terms of safety, adverse events (AEs) were well-balanced between the two arms. Grade 3/4 events possibly related to treatment occurred in 41% of patients in both arms. AEs leading to patients not undergoing RC occurred in 1% of patients in both groups. AEs delayed surgery in 2% of the Durvalumab arm and 1% of the patients in the comparator arm. Discontinuation of neoadjuvant chemotherapy (NAC) was 14% in the Durvalumab arm and 15% in the comparator arm and death occurred in 5% of patients in the Durvalumab group and 6% in the comparator arm.
The most frequently reported AEs were Nausea, Anaemia, constipation, and fatigue as illustrated below:
Dr. Powles concluded his presentation with the following take-home messages:
- The NIAGARA trial supports perioperative Durvalumab with NAC as a potential new standard treatment for patients with cisplatin-eligible (CrCl > 40 mL/min) MIBC.
- NIAGARA is the first Phase 3 perioperative immunotherapy study in MIBC and has demonstrated a statistically significant and clinically meaningful improvement in EFS (HR: 0.68, 95% CI: 0.56–0.82, p<0.0001) and OS (HR: 0.75, 95% CI: 0.59–0.93, p=0.0106).
- The pCR results and the significant OS benefit further support the perioperative approach.
- The addition of perioperative Durvalumab to NAC was tolerable, with no new safety signals. Most AEs were manageable, and Grade 3/4 events were similar in both treatment arms.
- Neoadjuvant Durvalumab did not delay surgery or impact the ability of patients to undergo or complete RC.
Presented by: Thomas B. Powles, MBBS, MRCP, MD, Department of Genitourinary Oncology, Barts Cancer Institute, Experimental Cancer Medicine Centre, Queen Mary University of London, St Bartholomew’s Hospital, London, UK
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003 Aug 28;349(9):859-66. doi: 10.1056/NEJMoa022148. Erratum in: N Engl J Med. 2003 Nov 6;349(19):1880. PMID: 12944571.
- Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, Ye D, Agerbaek M, Enting D, McDermott R, Gajate P, Peer A, Milowsky MI, Nosov A, Neif Antonio J Jr, Tupikowski K, Toms L, Fischer BS, Qureshi A, Collette S, Unsal-Kacmaz K, Broughton E, Zardavas D, Koon HB, Galsky MD. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114. doi: 10.1056/NEJMoa2034442. Erratum in: N Engl J Med. 2021 Aug 26;385(9):864. doi: 10.1056/NEJMx210012. PMID: 34077643; PMCID: PMC8215888.
- Andrea B. Apolo et al. AMBASSADOR Alliance A031501: Phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma (MIUC) vs observation. JCO 42, LBA531-LBA531(2024).
- Cathomas R, Rothschild SI, Hayoz S, Bubendorf L, Özdemir BC, Kiss B, Erdmann A, Aeppli S, Mach N, Strebel RT, Hadaschik B, Berthold D, John H, Zihler D, Schmid M, Alborelli I, Schneider M, Musilova J, Spahn M, Petrausch U. Perioperative Chemoimmunotherapy With Durvalumab for Muscle-Invasive Urothelial Carcinoma: Primary Analysis of the Single-Arm Phase II Trial SAKK 06/17. J Clin Oncol. 2023 Nov 20;41(33):5131-5139. doi: 10.1200/JCO.23.00363. Epub 2023 Aug 17. PMID: 37590894; PMCID: PMC10666980.


