(UroToday.com)The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Amanda Nizam presented an analysis of UNITE (Urothelial Cancer Network to Investigate Therapeutic Experiences) evaluating pre-treatment risk factors for enfortumab vedotin-induced peripheral neuropathy in patients with advanced urothelial carcinoma.
Enfortumab vedotin (EV) is a Nectin-4 targeted antibody-drug conjugate with an MMAE (microtubule-disrupting agent) payload. EV is approved as monotherapy for:
- Advanced urothelial carcinoma patients with disease progression following platinum and checkpoint inhibitor therapy
- Cisplatin-ineligible patients after ≥1 therapy line(s)
- Locally advanced/metastatic urothelial carcinoma in combination with pembrolizumab.
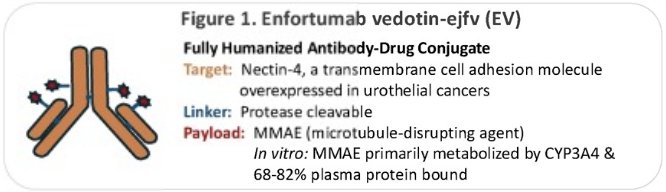
Peripheral neuropathy is a frequent, dose-limiting toxicity associated with EV, owing to cumulative exposure to MMAE. EV-associated peripheral neuropathy may therefore affect therapeutic exposure to EV, which can in turn influence treatment outcomes, and negatively affect quality of life. Knowledge on patient-specific risk factors for EV-associated peripheral neuropathy remains limited. Dr. Nizam and colleagues hypothesized that certain pre-treatment clinical factors may be associated with an increased risk for EV-associated peripheral neuropathy.
UNITE is a multi-institutional retrospective registry across 17 U.S. sites of patients with advanced urothelial carcinoma treated with targeted therapies including EV. Patient, disease, treatment, and toxicity-related data were obtained via a chart review at each site. Treatment-related adverse events were assessed per CTCAE v5.0.
A directed acyclic graph (DAG) was constructed to define causal relationships among clinical variables affecting the development of EV-associated peripheral neuropathy and to identify potential confounding factors. Associations between a priori-selected clinical factors at EV start and EV-associated peripheral neuropathy development were assessed using univariable analyses with logistic regression modeling and in separate multivariable (MVA) logistic regression models to adjust for confounding factors, as identified in the DAG.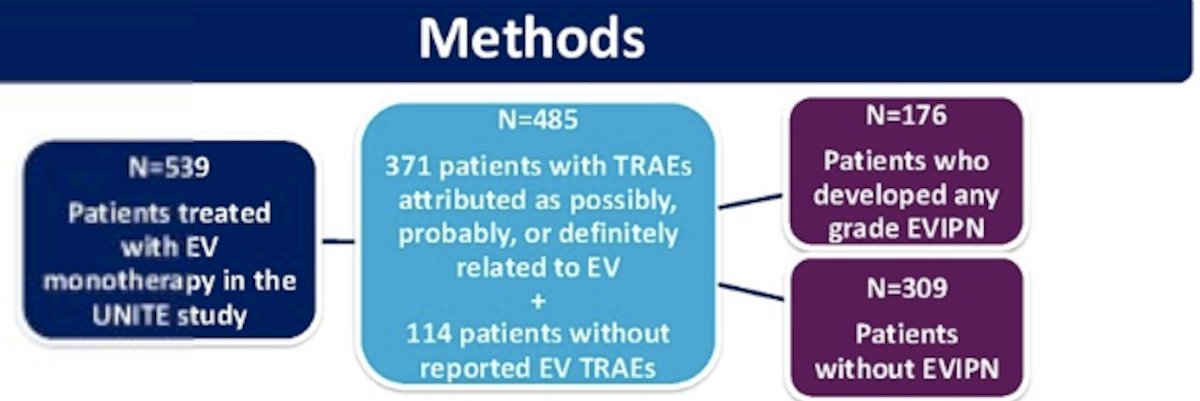
The study investigators identified 539 patients treated with EV monotherapy from the UNITE study, of whom 485 were study-eligible. Of these 485 patients, 371 experienced treatment-related adverse events attributed as possibly, probably, or definitely related to EV. 114 patients did not experience EV-related adverse events. Overall, 176 patients experienced any grade EV-associated peripheral neuropathy, while 309 patients did not.
The baseline patient demographics are summarized below: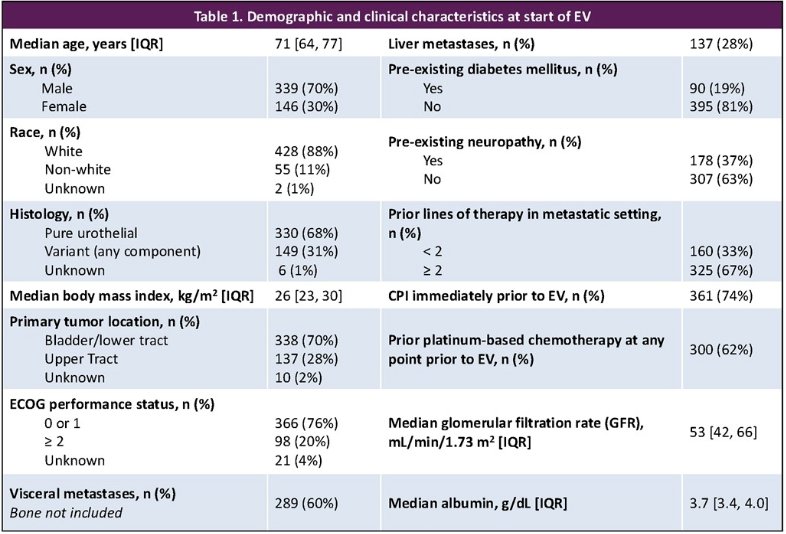
The median follow-up from EV start was 9.1 months, and the median time on EV was 4.1 months. The median time from EV start to neuropathy onset was 2.5 months (IQR: 1.2–4.6 months).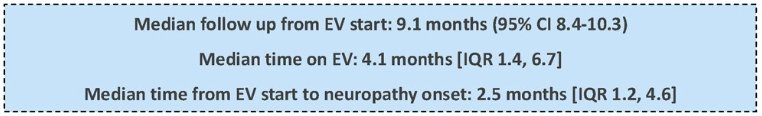
Of the 176 patients who experience EV-associated peripheral neuropathy, 20 were grade ≥3 in severity (11.4%). Of these 176 patients, 117 had any dose alteration event due to this neuropathy (24% of total cohort), including 45 (9% of total cohort) who permanently discontinued EV.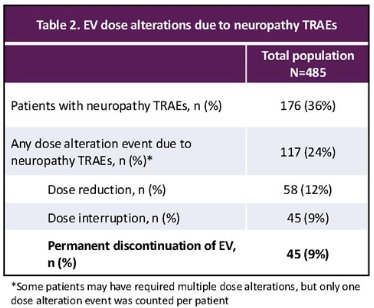
Illustrated below is the DAG of potential causative relationships between pre-treatment clinical factors and the development of EV-associated peripheral neuropathy.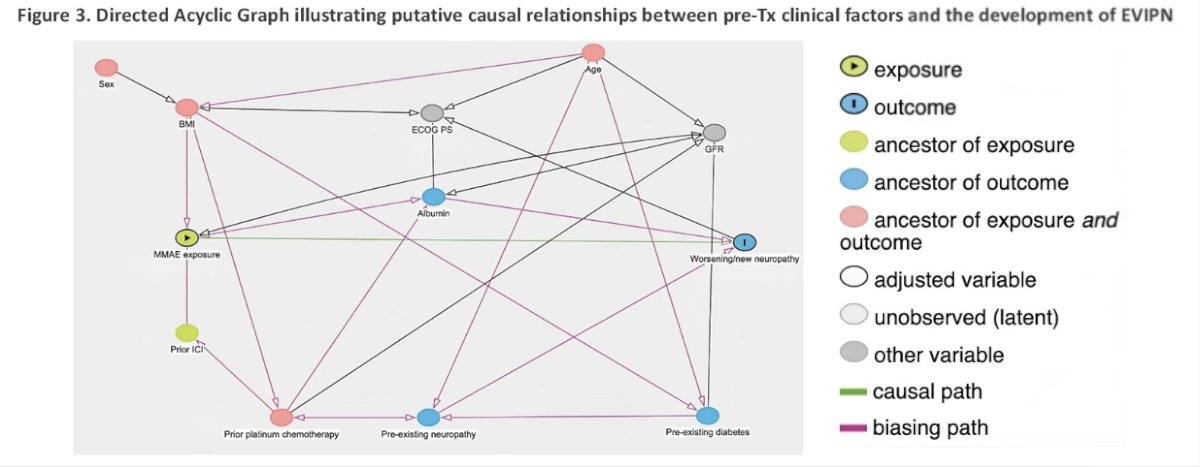
The univariable associations between clinical factors at EV start and the development of neuropathy are summarized below: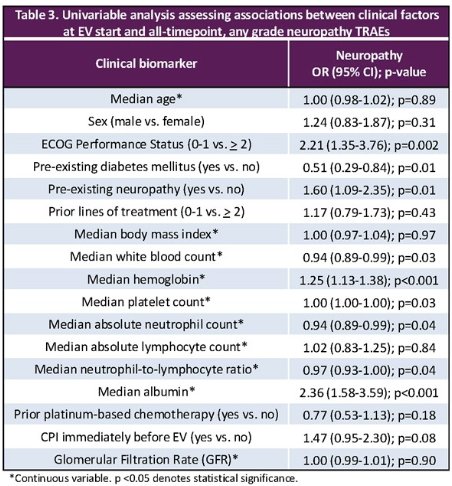
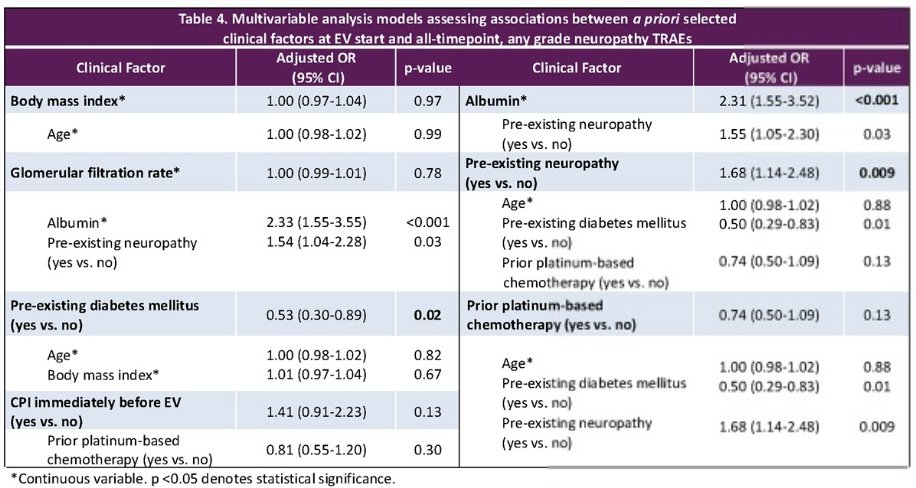
On multivariable modeling, the patient-level variables that were significantly associated with the odds of development of EV-associated peripheral neuropathy included:
- Pre-existing diabetes mellitus: OR=0.53, p=0.02
- Higher pre-treatment albumin levels: OR=2.31, p<0.001
- Pre-existing neuropathy: OR=1.68, p=0.009
Dr. Nizam concluded as follows:
- A multivariable analysis of EV-treated patients with advanced urothelial carcinoma identified putative pre-treatment factors associated with a higher risk for EV-induced peripheral neuropathy, including higher pre-treatment albumin levels and pre-existing neuropathy.
- The association between pre-existing diabetes mellitus and EV-induced peripheral neuropathy requires further elucidation due to the limited population of patients with pre-existing diabetes mellitus in this study,
- These hypothesis-generating results suggest closer monitoring and earlier intervention to mitigate EV-induced peripheral neuropathy may be warranted for certain patients starting EV, including those with higher pre-treatment albumin levels and pre-existing neuropathy.
- EV-induced peripheral neuropathy is a frequent dose-limiting toxicity that can affect cumulative therapeutic exposure. and thus treatment outcomes, while also negatively impacting quality of life.
- Prospective studies to identify and validate risk factors for EV-induced peripheral neuropathy are critical for optimizing outcomes in patients with advanced urothelial carcinoma.
Presented by: Amanda Nizam, MD, Hematology and Medical Oncology, Cleveland Clinic, Cleveland, OH
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


