(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a genitourinary cancers poster session. Dr. Guarionex DeCastro presented the results of a phase II trial of intravesical cabazitaxel, gemcitabine, and cisplatin for the treatment of BCG-unresponsive non-muscle invasive bladder cancer (NMIBC).
In high-risk NMIBC patients, up to 40% will relapse after BCG, with limited available salvage intravesical therapies available. In 2013, Dr. DeCastro and colleagues conducted a preclinical trial in a p53/PTEN genetically engineered mouse model of high-grade bladder using intravesical multidrug chemotherapy.

In 2019, a phase I study of 18 patients demonstrated the safety of a novel, sequential intravesical combination regimen of cabazitaxel, gemcitabine, and cisplatin.
The inclusion criteria for this phase II study were:
- Age 18 years or older
- Histologically confirmed high grade Ta, high or low grade T1, or CIS with/without Ta or T1
- BCG refractory or BCG recurrent/relapsing
- Prostatic urethral involvement and history of receiving second-line intravesical therapies were allowed
The exclusion criteria were as follows:
- Grade ≥2 peripheral neuropathy
- Pregnancy
- Evidence of upper tract disease
Systemic and local dose-limiting toxicities were defined using the National Cancer Institute Common Toxicity Criteria (version 4.0). Induction with cabazitaxel, gemcitabine, and cisplatin included weekly instillations of gemcitabine and cabazitaxel (on the same day) and gemcitabine every other week (on a different day).
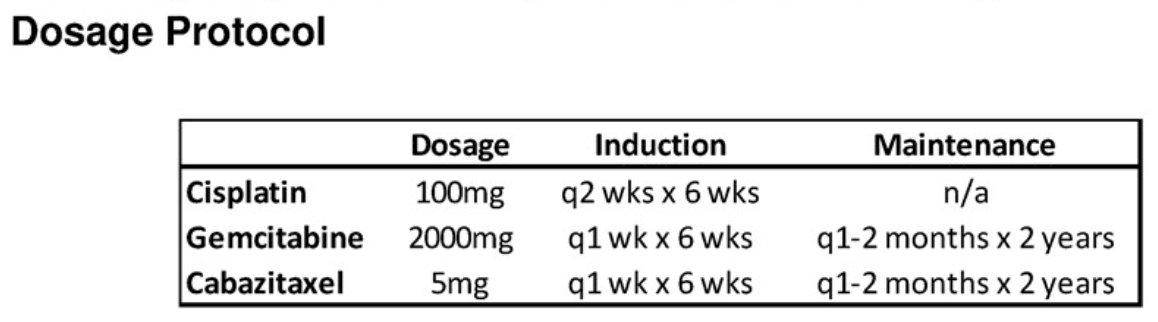
The surveillance protocol was as follows:
- Obligatory rigid cystoscopy with biopsy at 3 months (after completion of induction)
- Usage of Cysview was not required but was used in the majority of subjects
- Freedom from recurrence was defined as no visible tumors on cystoscopy and biopsies negative for high grade disease
- Subjects without evidence of recurrence continued on maintenance for two years
- Cabazitaxel and gemcitabine were administered monthly for the 1st year, then every other month for the 2nd year
- Cisplatin not administered during maintenance
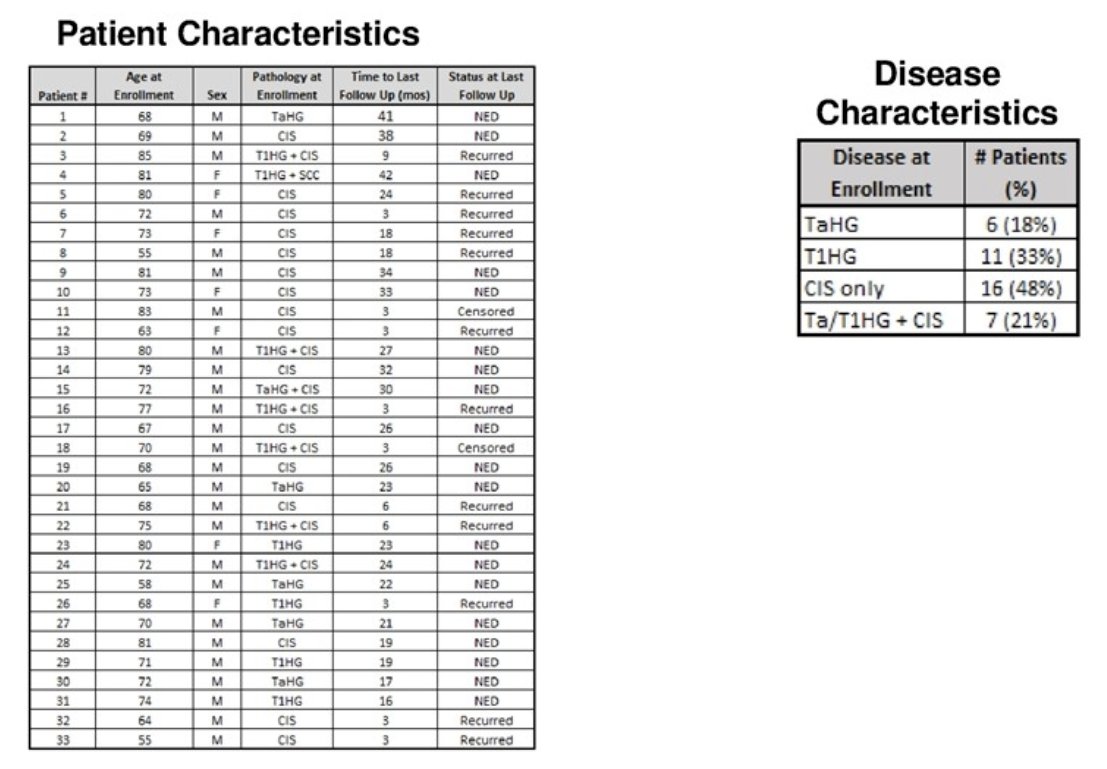
This trial enrolled 33 patients, of whom 7 were female. The mean patient age was 72 years. The minimum follow-up duration for all patients was 15 months. The median follow-up was 20 months (range: 17 – 43 months). The patient characteristics are summarized below. At enrolment, 69% of patients had CIS +/- papillary disease. The remainder had papillary-only disease.
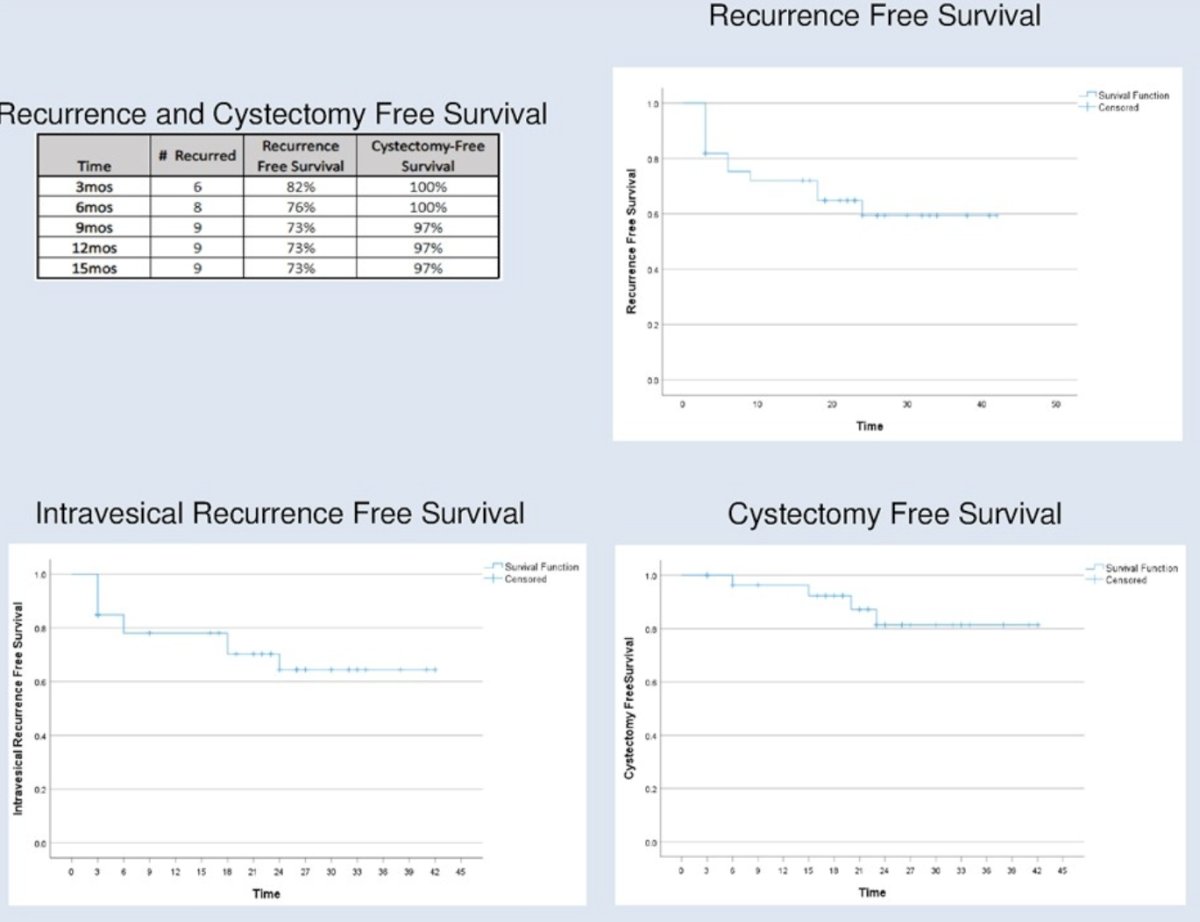
The recurrence-free survivals at 3 and 12 months were 82% and 73%, respectively. Two recurrences were within the prostatic urethra. The median recurrence-free survival was 34 months. The one-year cystectomy-free survival rate was 97%.
Dr. DeCastro concluded as follows:
- The intravesical combination of cabazitaxel, gemcitabine, and cisplatin is a promising salvage intravesical option in a high-risk cohort of BCG-unresponsive patients
- Toxicities were limited to local side effects, and none were dose-limiting
- At 3 months post-induction, 82% of patients had no evidence of recurrence. At 15 months, the latest time point for which data was available for all patients, 73% had no evidence of recurrence. At this same time point, 96% of patients remained cystectomy-free. The estimated median recurrence free survival was 34 months.
Presented by: Guarionex J. DeCastro, MD, Assistant Professor, Department of Urology, Columbia University Medical Center, New York, NY
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Reference:
- DeCastro GJ, Sui W, Pak JS, et al. A Phase I Trial of Intravesical Cabazitaxel, Gemcitabine and Cisplatin for the Treatment of Nonmuscle Invasive bacillus Calmette-Guérin Unresponsive or Recurrent/Relapsing Urothelial Carcinoma of the Bladder. J Urol. 2020; 204(2):247-53.


