(UroToday.com) The 2024 ESMO annual meeting included a session highlighting urothelial carcinoma trials in progress, with Dr. Tian Zhang discussing STAR-EV, a phase I/II study of stereotactic treatment with neoadjuvant radiotherapy and enfortumab vedotin for localized cisplatin ineligible, muscle invasive bladder cancer. Urothelial cancer is the 9th most common cancer worldwide, with more than 610,000 people diagnosed per year. Muscle invasive bladder cancer needs more treatment options, and up to 50% of patients with muscle invasive bladder cancer cannot receive cisplatin-based chemotherapy. Definitive treatment of muscle invasive bladder cancer depends on neoadjuvant chemotherapy and surgery, or trimodality therapy with maximal resection followed by chemoradiation therapy.
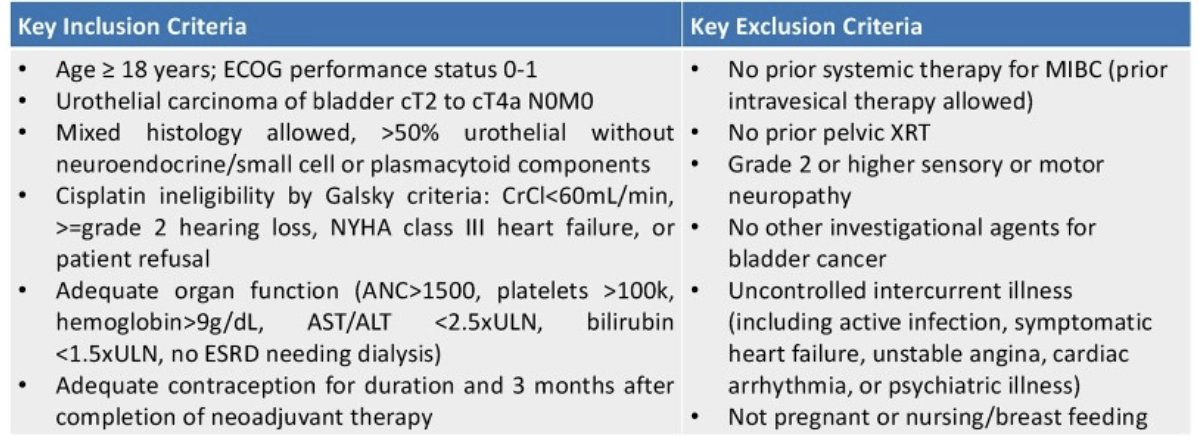
Enfortumab vedotin is an antibody drug conjugate targeted to nectin-4 and delivers MMAE; enfortumab vedotin is now standard of care in metastatic urothelial carcinoma.1 Based on EV-103 cohort H, patients with localized, cisplatin ineligible muscle invasive bladder cancer respond well to enfortumab vedotin, with 36% pathologic complete responses noted at radical cystectomy. Radiation is also an effective therapy for muscle invasive bladder cancer, and a retrospective patient series treated with enfortumab vedotin and radiation showed overall safety. Therefore, Dr. Zhang and colleagues designed a trial with enfortumab vedotin and radiation to improve pathologic complete response rates. The key inclusion and exclusion criteria are as follows:
This is a single center, phase 1/2 trial based at UT Southwestern Medical Center. Patients will receive enfortumab vedotin 1.25 mg/m2 IV days 1/8 every 3 weeks for 3 cycles, with either sequential or concurrent stereotactic body radiation (SBRT) in 5 fractions. The safety lead-in phase will consist of SBRT given at C3 day 21 and then escalated forward to start at C2 day 15 (level 1) or C1 day 15 (level 2). All patients will then undergo radical cystectomy. The trial design is as follows:
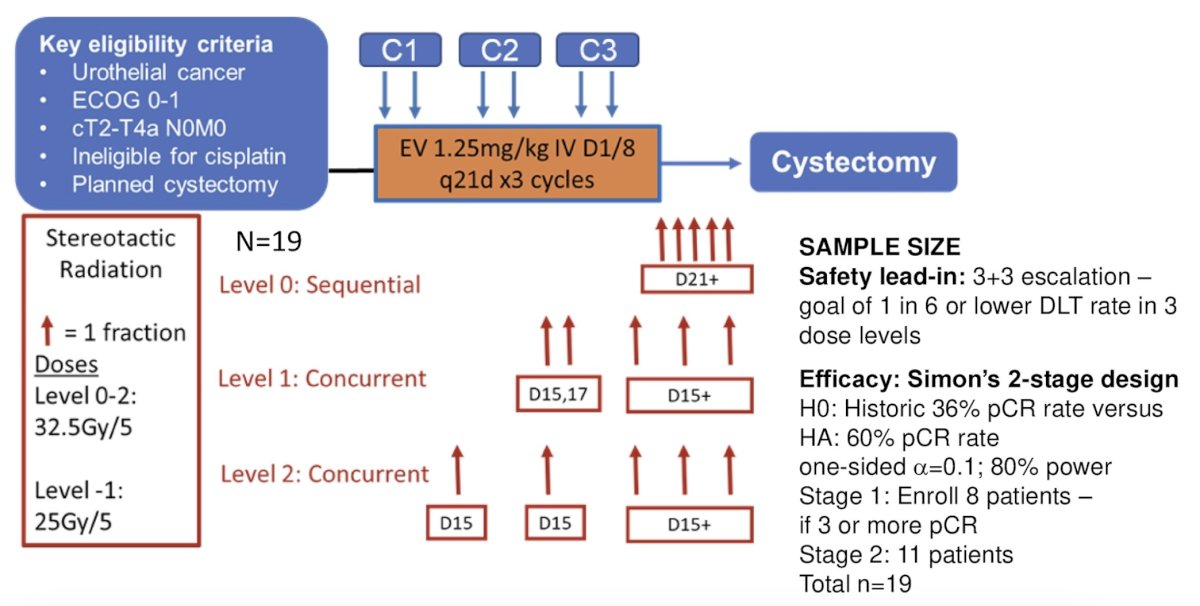
Dose limiting toxicities during the safety portion include non-hematologic adverse events grade 3 or higher, non-completion of either 3 cycles of enfortumab vedotin, delay of SBRT over 2 weeks, or delay of radical cystectomy over 8 weeks. Rate of pathologic complete response is the primary endpoint for efficacy, with a goal of 60% pathologic complete response. In a Simon’s two-stage design, if ≥3 pathologic complete responses are seen in the first 8 patients, 11 additional patients will be enrolled, for a total of 19. The null hypothesis will be rejected if ≥10 pathologic complete responses are seen. Secondary endpoints include:
- Safety of the enfortumab vedotin and SBRT
- Rate of pathologic down-staging
Exploratory objectives include:
- Quality of life
- Disease free survival after radical cystectomy
- Delay of radical cystectomy >8 weeks from the end of enfortumab vedotin/SBRT
As enfortumab vedotin improves metastatic urothelial carcinoma outcomes, there is an opportunity to improve localized disease treatments for muscle invasive bladder cancer. Improving pathologic complete response rates with enfortumab vedotin and radiation will be a hypothesis generation for future trials in localized bladder cancer. Serum and urinary biomarkers will be explored. The study is IND-exempt and will be open and enrolling (clinicaltrials.gov: NCT06394570).
Presented by: Tian Zhang, MD, UT Southwestern University, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: Enfortumab Vedotin and Stereotactic Radiation Tested Before Surgery in Muscle-Invasive Bladder Cancer in STAR-EV Trial - Tian Zhang
References:


