(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session presentation of Poster 2016. Dr. Cora N. Sternberg discussed the evaluation of event-free survival (EFS) as a surrogate endpoint for overall survival (OS) in muscle-invasive bladder cancer (MIBC) following neoadjuvant therapy.
The standard of care for muscle-invasive bladder cancer (MIBC) remains radical cystectomy with cisplatin-based neoadjuvant chemotherapy for eligible patients. However, overall survival (OS) readouts can take over five years to mature. Event-free survival (EFS) is a common intermediate endpoint in neoadjuvant/perioperative oncology trials, allowing for accelerated treatment evaluation while awaiting OS results. Additionally, EFS may be preferred when the investigator wants the endpoint to reflect the primary treatment, rather than subsequent treatments given if the study drug fails or if relapse occurs.
Dr. Sternberg and colleagues evaluated EFS as a clinical surrogate endpoint for OS in neoadjuvant therapy-treated MIBC patients. They conducted a systematic literature review to identify clinical trials for neoadjuvant therapy before radical cystectomy in MIBC (stage T2-T4a, N0-N1, M0) reporting both EFS and OS outcomes published until June 7, 2023. Six databases (ie, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Northern Light Life Sciences Conference Abstracts database, and the Database of Abstracts of Reviews of Effects) were searched using keywords related to MIBC, neoadjuvant treatments, and relevant survival outcomes (EFS and OS). Study eligibility criteria for the systematic review is outlined below: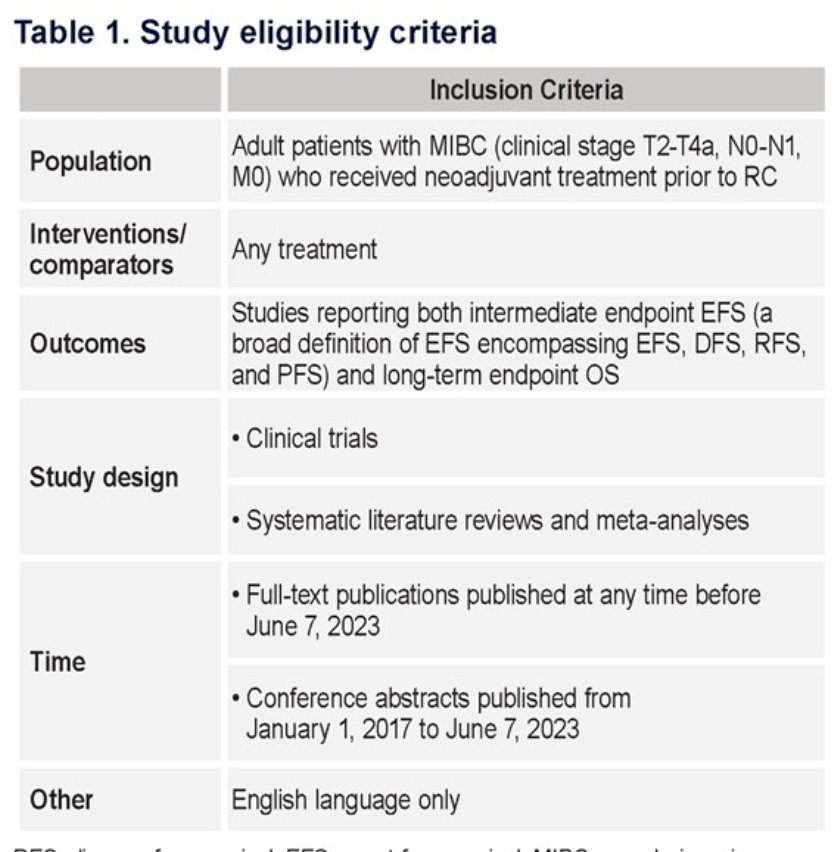
They used a broad definition of EFS encompassing EFS, progression-free survival (PFS), recurrence/relapse-free survival (RFS), and disease-free survival (DFS), all of these definitions were considered in the systematic review, and the underlying definitions of these endpoints in each study were scrutinized to confirm relevance.
The trial-level surrogacy was evaluated by assessing the association of hazard ratio (HR) of EFS with the HR of OS; outcome-level surrogacy was evaluated by assessing the associations of survival rates and median survival time between EFS and OS, respectively.
They meta-analyzed the associations between HR of EFS with HR of OS using linear regression weighted by study sample size. The strength of the association was measured by coefficient of determination (R2) which is a statistical measure of how well the regression predictions approximate the real data points. An R2 of 1 indicates that the regression predictions perfectly fit the data. Additionally, they calculated reported surrogate threshold effect (STE) indicating the minimum HR of EFS needed to predict a significant HR of OS <1. (1)
Dr. Sternberg and colleagues identified a total of 390 publications, of which 15 trials met the eligibility criteria and were included in this study. These comprised 6 randomized controlled trials (RCTs) encompassing a total of 1,948 patients, and 9 single-arm trials. The analyses of outcome-level surrogacy included 21 study arms from 15 trials with a total of 2,509 patients. The majority of trials were conducted among patients with N0 (node-negative) tumors, with median patient age ranging from 63 to 70 years. The neoadjuvant treatments used in the trials included cisplatin- or carboplatin-based chemotherapy alone (n=12), chemotherapy in combination with a targeted therapy (n=2), and immunotherapy (n=1). The study selection schema is shown below: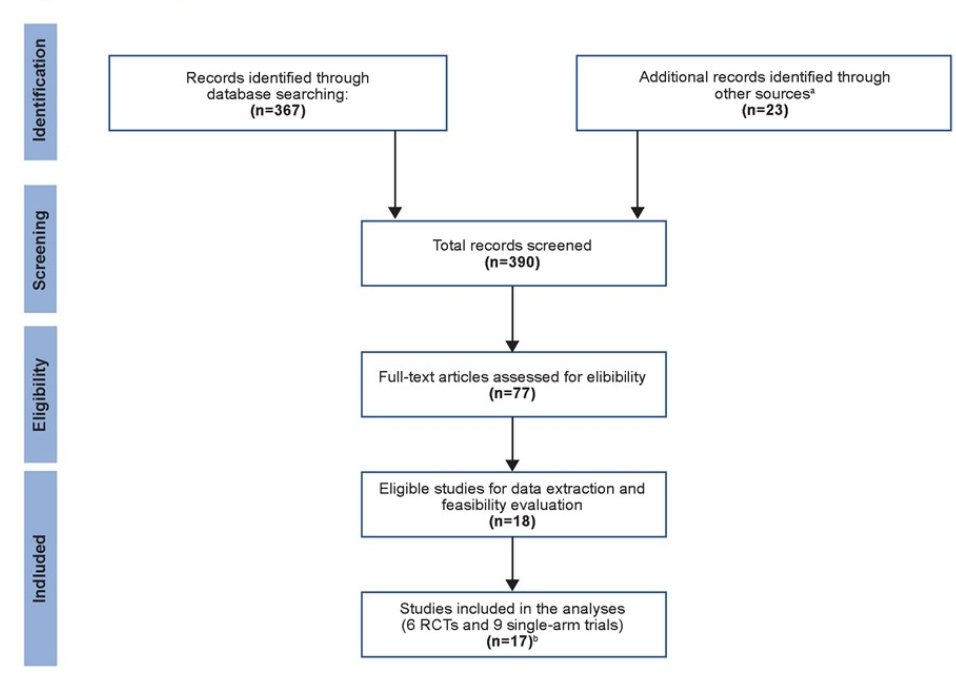
Six eligible RCTs were included in the evaluation of the treatment effect association between EFS and OS, with a total sample size of 1,948 patients. The treatment effect of EFS was significantly associated with the treatment effect of OS, with an estimated coefficient of 1.26 (P=0.001) and an R² of 0.94 (95% CI, 0.74-1.00). An R² of 0.94 indicates that 94% of the variance in the HR of OS can be predicted by the HR of EFS from the regression model. The surrogate threshold effect (STE), which was the minimum HR of EFS needed to predict a significant HR of OS <1, was 0.88. These results are illustrated in the graphic below: 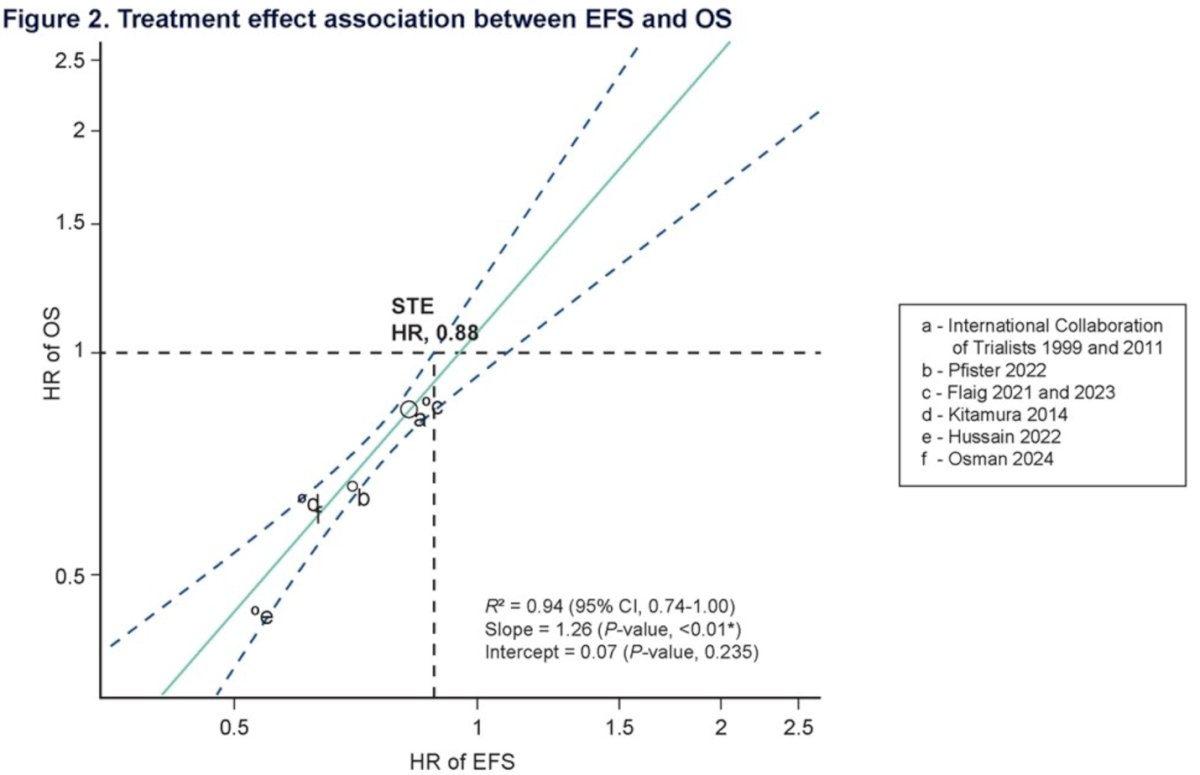
Consistent associations between EFS and OS were observed in the sensitivity analyses, with R2 of 0.91, 0.91, and 0.87, for the 3 sensitivity analyses:1 among studies of stage T2-4N0M0,2 studies using cisplatin-based chemotherapy as neoadjuvant therapy,3 among studies using RC as definitive treatment, respectively.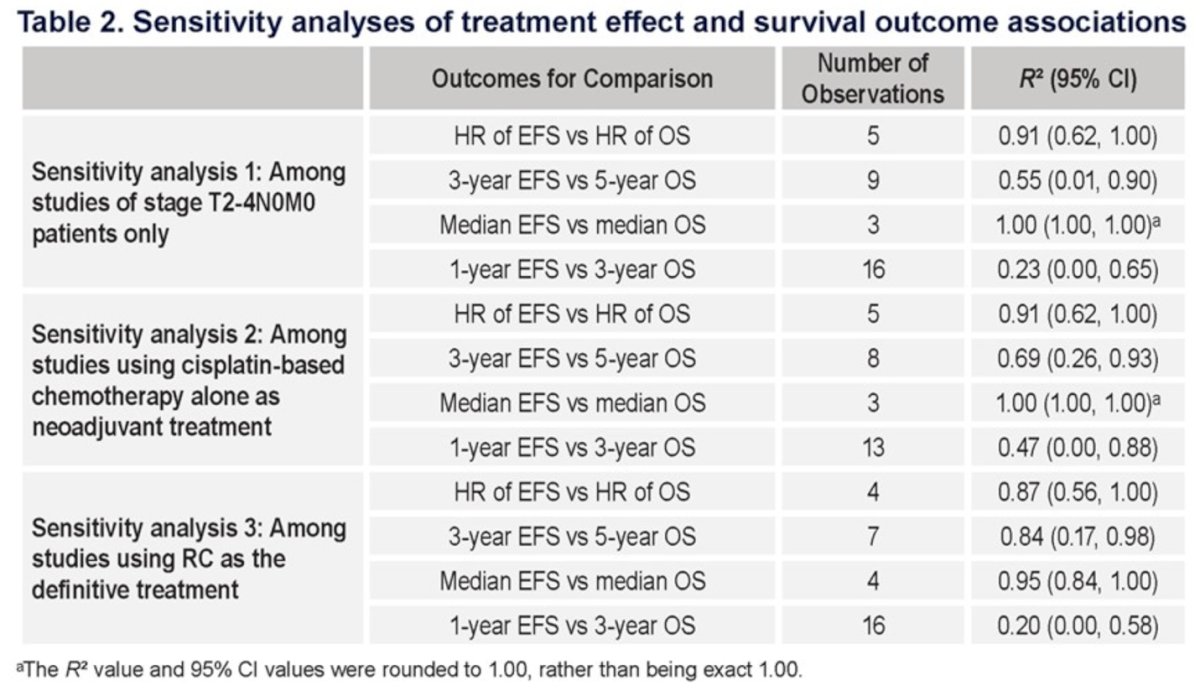
Furthermore, they evaluated outcome-level surrogacy between 3-year EFS versus 5-year OS, median EFS vs. median OS, and 1-year EFS vs. 3-year OS. Significant outcome-level associations were observed between 3-year EFS and 5-year OS, including 12 study arms for this analysis, the R2 was 0.84 (95% CI 0.28, 0.95) with a p-value of <0.001.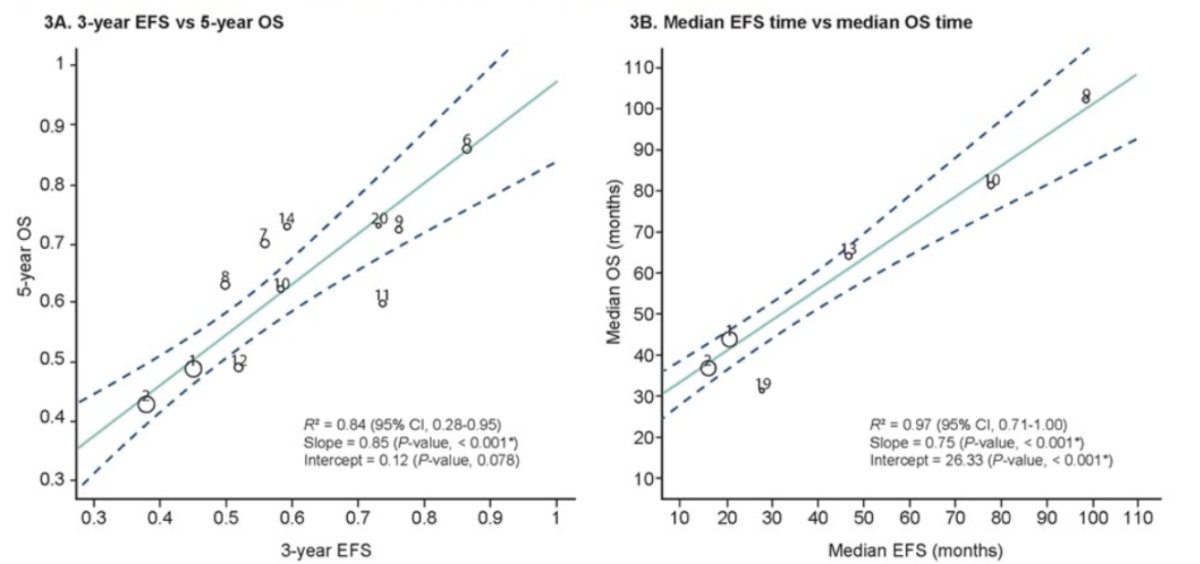
Consistently, the median EFS time and median OS time analysis showed significant outcome-level associations R2 0.97 (0.71, 1.00) as well as 1-year EFS and 3-year OS, with R2 0.70 (0.10, 0.89).

Lastly, the 1-year EFS was significantly associated with 3-year OS (P<0.001), with an R2 of 0.70 (95% Cl,0.10-0.89);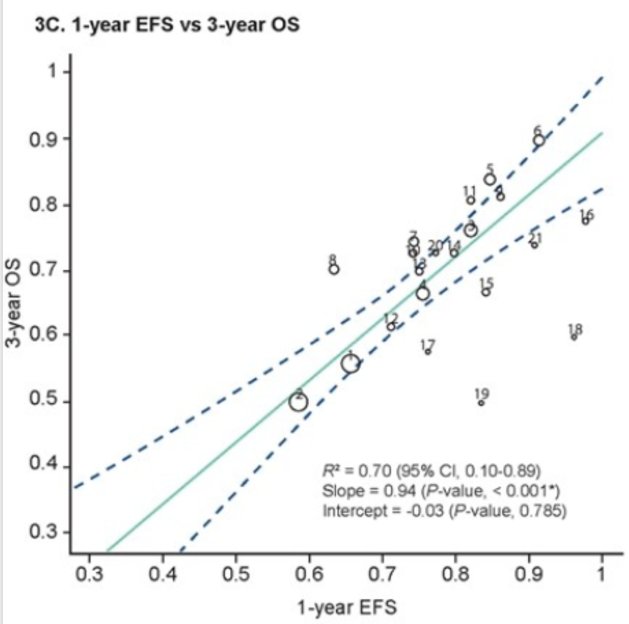
Dr. Sternberg concluded her presentation delivering the following key takeaways:
- There is a strong association between EFS and OS in terms of treatment effects (R2 of 0.94) and survival outcome measure
- Significant outcome-level associations were observed between 3-year EFS vs. 5-year OS, median EFS vs. median OS, and 1-year EFS vs. 3-year OS in clinical trials for MIBC following neoadjuvant therapy.
- The study findings support the use of EFS as a surrogate endpoint for OS for treatment evaluation in the Neoadjuvant setting for MIBC.
Presented by: Cora N. Sternberg, MD, Professor of Medicine, Clinical Director of the Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related content: Event-Free Survival as Surrogate Endpoint in Muscle Invasive Bladder Cancer Research - Cora Sternberg
- Di Bucchianico, Alessandro. "Coefficient of determination (R 2)." Encyclopedia of statistics in quality and reliability (2008).


