(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the Proffered Paper session 2: GU, non-prostate. Dr. Andromachi Kougioumtzopoulou presented the findings of a a phase II, randomized study by the Hellenic GU Cancer Group exploring Nivolumab plus chemoradiotherapy in patients with non-metastatic muscle-invasive bladder cancer, not undergoing cystectomy.
There is ongoing interest in bladder-sparing strategies for muscle-invasive bladder cancer (MIBC). Trimodal therapy (TMT) specifically involves maximal transurethral resection of the bladder tumor, followed by a combination of chemotherapy and radiation therapy (RT). Initial data from both prospective and retrospective studies, including the BC200 and BCON trials, demonstrated that chemotherapy with fluorouracil and mitomycin C, when combined with radiotherapy, significantly improved locoregional control of bladder cancer compared to radiotherapy alone.1 However, challenges in patient enrollment have led to some TMT trials closing prematurely due to slow accrual. Recently, Dr. Alexandre Zlotta from the University of Toronto, published a propensity score-matching analysis using data from academic centers in North America. This analysis included 837 radical cystectomy and 282 TMT patients. The study found no significant differences in metastasis-free survival (MFS), cancer-specific survival (CSS), or disease-free survival (DFS) between TMT and radical cystectomy (RC). Notably, overall survival was slightly better with TMT (68% vs. 73%), and the hazard ratio (HR) was 0.75 (95% CI, 0.58-0.97; p=0·0078).2
The rationale for employing immunotherapy in non-metastatic muscle-invasive bladder cancer (MIBC) stems from its success in treating metastatic urothelial carcinoma with anti-PD-1 and anti-PD-L1 agents. Nivolumab, an anti-PD-1 therapy, has been approved as adjuvant treatment for high-risk MIBC following radical cystectomy (RC). Preliminary data also suggest a potential synergistic effect when combining immunotherapy with radiotherapy.3 However, the role of immunotherapy for MIBC patients who have not undergone RC is still under investigation, particularly in the neoadjuvant or the perioperative setting (e.g., the NIAGARA trial) and in combination with radiotherapy. Dr. Kougioumtzopoulou and her team are exploring the impact of concurrent nivolumab and chemoradiotherapy in MIBC patients who either did not undergo RC or were deemed unfit for the procedure.
This study enrolled patients with treatment-naïve muscle-invasive bladder cancer (MIBC), classified as cT2-T4aN0M0, with histologies including urothelial, squamous, or glandular types, an ECOG performance status (PS) of ≤ 1, and a glomerular filtration rate (GFR) of ≥ 40 ml/min. All participants had previously undergone "vigorous" transurethral resection of the bladder tumor (TURBT). Patients were randomized to receive either chemoradiotherapy (ChemoRT) for 6 or 7 weeks, consisting of Cisplatin-based chemotherapy 20 mg daily from days 1 to 5 and days 22 to 25, or 40 mg/m² weekly for 6 weeks, or the experimental arm receiving nivolumab 240 mg intravenously every 14 days for 5 cycles, starting 14 days prior to ChemoRT, followed by 480 mg every 28 days for 6 cycles, in addition to Cisplatin 20 mg daily from days 1 to 5 and days 22 to 25, or 40 mg/m² weekly for 6 weeks. All patients received the same RT (3DCRT or IMRT)
Schedule to the whole bladder 60 Gy with a boost of 10 Gy to the tumour side (2 Gy/fraction). The primary endpoint of the study was locoregional recurrence-free rate (LRFR). The study design is shown below: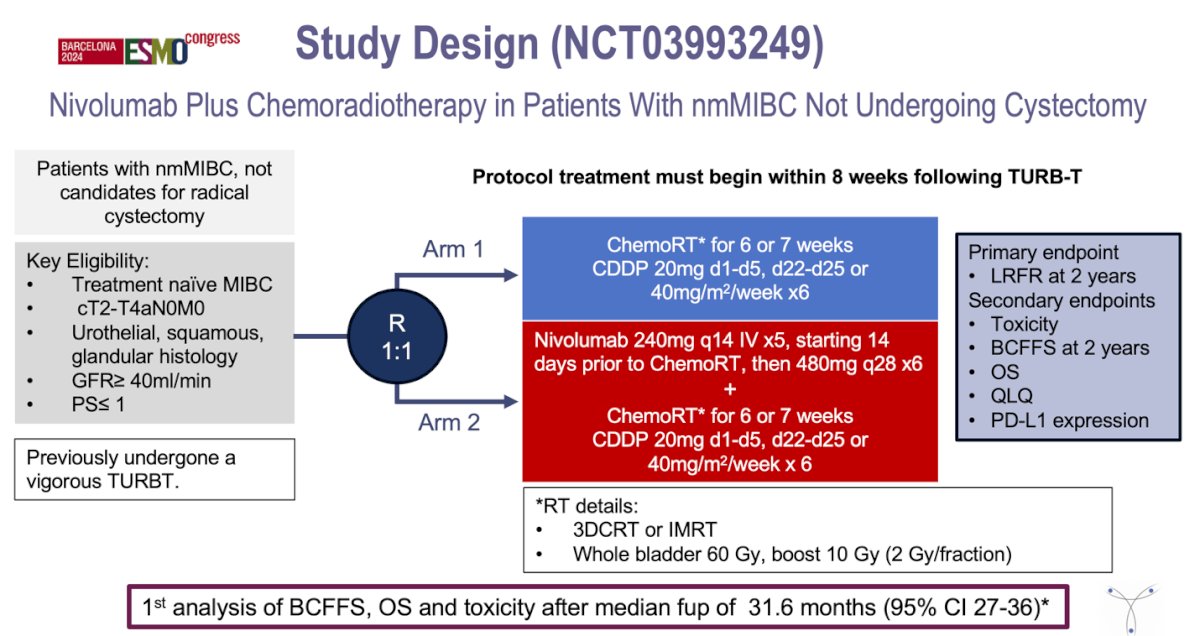
A total of 77 patients were randomized between June 2019 and October 2022. The baseline characteristics were well balanced, with the exception of a higher GFR in the control arm (0.014), the baseline and key demographic characteristics are summarized in the table below: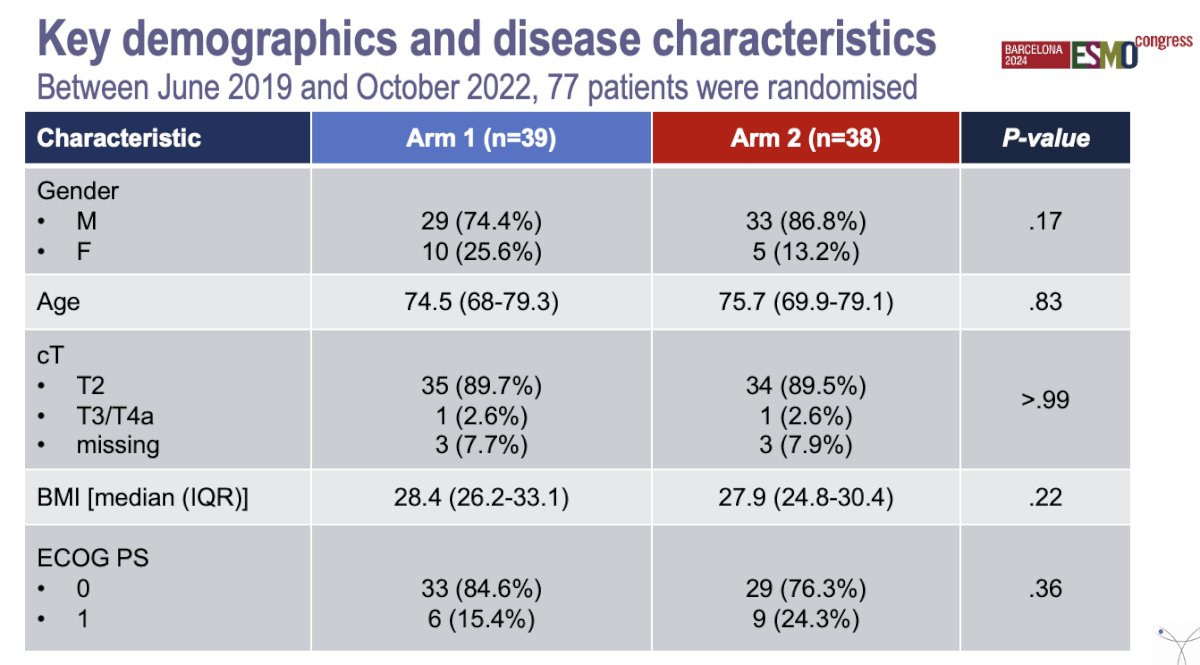
Compliance with the RT schedule was outstanding, with 87.2% in the control arm versus 84.2% in the experimental arm. There were 21 (53.8%) locoregional relapses in the control arm compared to 12 (31.6%) in the Nivolumab + TMT arm. Additionally, distant relapses occurred in 25.6% of the control arm and 28.9% of the experimental arm. The investigators documented 15 (38.5%) fatalities in the control arm versus 8 (21.1%) in the experimental arm.
The 2-year rate of bladder cancer failure-free survival was 42.7% in the TMT arm vs. 68.6% in the TMT +Nivolumab arm, this difference was significant (p=0.021).
The OS data was still immature for both groups, but the 2-year OS was 62.3% for the control arm vs. 88.6% for the experimental arm, this preliminary data was significant (p=0.013).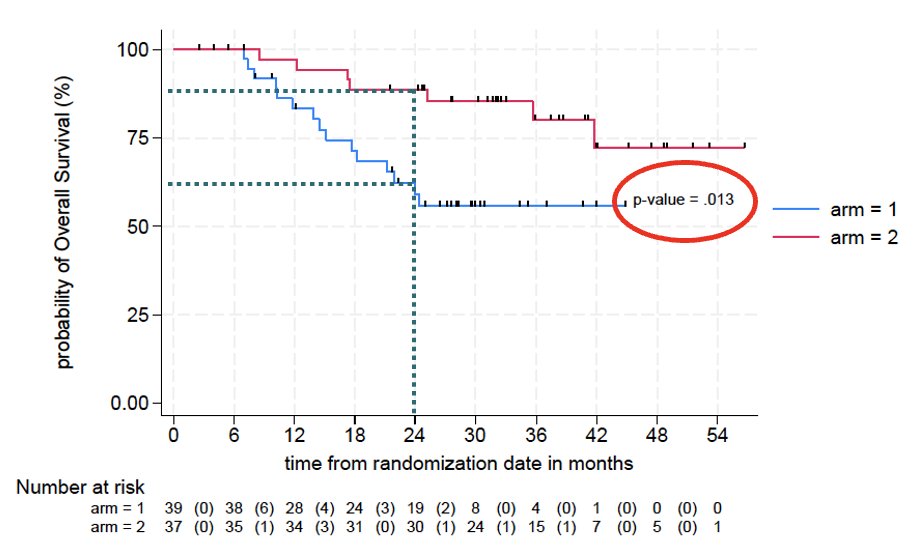
Toxicity was more common in the experimental arm, occurring in 67.2% of patients compared to 32.8% in the control arm, a difference that was statistically significant. However, the incidence of Grade >3 adverse events was similar between the two arms, 10 (25.6%) vs. 14 (36.8%), p=0.29. The most common Grade >3 adverse events are detailed in the table on the right.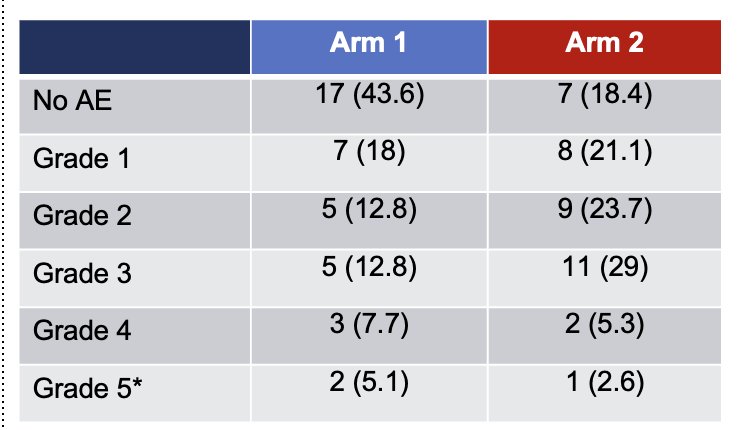
Three patients died during study treatment. No death was treatment-related: 1 due to COVID-19 infection and 2 due to disease progression.
Dr. Kougioumtzopoulou wrapped up her presentation with the following messages:
- The addition of nivolumab to TMT produced a significant increase in bladder cancer failure-free survival and OS in patients with MIBC not undergoing RC.
- The prolongation of bladder cancer failure-free survival and OS resulted from a significant decrease in the occurrence of distant metastases and locoregional relapses in patients receiving Nivolumab +TMT.
- The combination of TMT+ Nivolumab was well tolerated without any new safety signals observed, no difference in Grade >3 AEs
- These results support the conduction of a randomized phase III trial to establish nivolumab plus TMT as a new standard for patients with MIBC not undergoing RC.
Presented by: Andromachi Kougioumtzopoulou MD, Radiotherapy Unit, 2nd Department of Radiology, Medical School, National and Kapodistrian University of Athens, Rimini 1, 124 62 Athens, Greece.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA; BC2001 Investigators. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012 Apr 19;366(16):1477-88. doi: 10.1056/NEJMoa1106106. PMID: 22512481.
- Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, Drumm M, Mari A, Thio E, Fleshner NE, Kulkarni GS, Jewett MAS, Bristow RG, Catton C, Berlin A, Sridhar SS, Schuckman A, Feldman AS, Wszolek M, Dahl DM, Lee RJ, Saylor PJ, Michaelson MD, Miyamoto DT, Zietman A, Shipley W, Chung P, Daneshmand S, Efstathiou JA. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6):669-681. doi: 10.1016/S1470-2045(23)00170-5. Epub 2023 May 12. PMID: 37187202.
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014 Nov 27;515(7528):558-62.


