(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the Proffered Paper session 2: GU, non-prostate. Dr. Jens Bedke presented the results of an exploratory analysis of the CheckMate 901 trial of nivolumab as first-line therapy for unresectable or metastatic urothelial carcinoma, exploring health-related quality of life.
Platinum-based chemotherapy has long been the standard of care (SOC) for previously untreated, unresectable, or metastatic urothelial carcinoma (mUC), with cisplatin-based regimens preferred for cisplatin-eligible patients. The Phase 3 CheckMate 901 trial assessed the efficacy of upfront combination therapy with Nivolumab + Gemcitabine/Cisplatin (GC), followed by Nivolumab monotherapy, versus GC alone in cisplatin-eligible patients with previously untreated unresectable or mUC.1 The trial demonstrated that Nivolumab + GC significantly improved overall survival (OS) (HR, 0.78; 95% CI, 0.63-0.96; P = 0.02) and progression-free survival (PFS) (HR, 0.72; 95% CI, 0.59-0.88; P = 0.001). The overall response rate (ORR) was 57.6% with Nivolumab + GC compared to 43.1% with GC alone, and deep, ongoing complete remissions were observed in 21.7% of patients receiving Nivolumab + GC versus 11.8% with GC alone. (1)These results led to the approval of Nivolumab + GC for the first-line treatment of patients with unresectable or mUC by both the US FDA in March 2024 and the European Commission in May 2024.
Dr. Bedke presented an exploratory analysis of the treatment effects of adding Nivolumab to GC on patient-related outcomes (PRO) and health-related quality of life (HRQoL) of patients in the CheckMate 901 trial.
Recapitulating the study design schema for CheckMate 901, 608 patients underwent 1:1 randomization, stratified by tumor PD-L1 expression and presence/absence of liver metastases, to either:
- Nivolumab 360 mg on D1 + gemcitabine (1,000 mg/m2) on D1/8 + cisplatin (70 mg/m2) on D1 in 3-week cycles, up to a total of 6 cycles. Followed by: Nivolumab maintenance at 480 mg every 4 weeks (continued as maintenance therapy in responders until progression, unacceptable toxicity, withdrawal, or up to 24 months.)
- Gemcitabine + cisplatin at the same doses/schedule/cycles
The co-primary endpoints were OS and progression-free survival (PFS), per blinded, independent, central review (BICR). Key secondary endpoints included HRQoL and PRO.

All randomized patients with an evaluable PRO assessment at baseline and ≥ 1 evaluable post-baseline assessment were included in this analysis. The PRO assessment schedule was split into two phases:
- Combination phase (Nivolumab+GC and GC arms): cycle 1 day 1, then every other 3-week cycle for the first 6 months
- Monotherapy phase (Nivolumab+GC arm only): every 4 weeks, then every 12 weeks thereafter
The PRO measures utilized different HRQoL metrics and questionnaires and was divided in primary and secondary:
- Primary: global health status/QoL (EORTC QLQ-30)
- Secondary:
- Physical functioning (EORTC QLQ-C30)
- Role functioning (EORTC QLQ-C30)
- Fatigue (EORTC QLQ-C30)
- EQ-VAS (EQ-5D-5L)
The statistical analysis of this exploratory analysis of CheckMate 901 included a primary analysis measuring the change from baseline to week 16 in EORTC QLQ-C30 global health status using a linear mixed-effect model for repeated measures (MMRM). Additional analyses explored the MMRM change for remaining EORTC QLQ-C30 domains and EQ-VAS from baseline to week 16, completion rates of the questionnaires over time by arm, change over time for all domains, and the percentage of patients with clinically meaningful improvement or deterioration from baseline to week 16 for all domains. A noninferiority design was used, with predefined margins based on prior data from PRO/HRQoL.
There were 276 PRO-evaluable patients in the Nivolumab + GC arm and 248 in the GC arm. The groups were well-balanced. Most patients had a bladder tumour (77.5% and 72.2%). Other characteristics are shown below:
Completion rates during the combination phase were comparable between the Nivolumab + GC arm and the GC arm. Notably, the completion rates remained ≥ 60%, except at week 16 for Nivolumab + GC. This could be explained by the original design of the CheckMate 901 trial, which was intended to explore Nivolumab + Ipilimumab vs. GC. The Nivolumab + GC arm was added later, potentially altering the dose schedule of Nivolumab + GC compared to Ipilimumab + Nivolumab. Additionally, in some centers, PRO measurements were not conducted at week 16.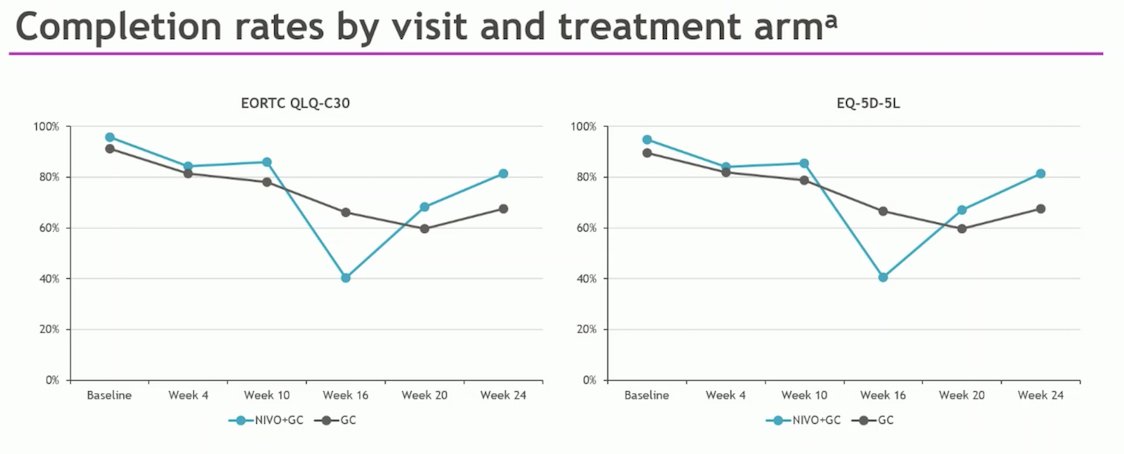
The baseline PRO scores (in the table below) were comparable between the two arms and consistent with the literature on similar populations. Dr. Bedke mentioned that they did not expect an improvement in PRO scores with the addition of Nivolumab, hence the use of a noninferiority design.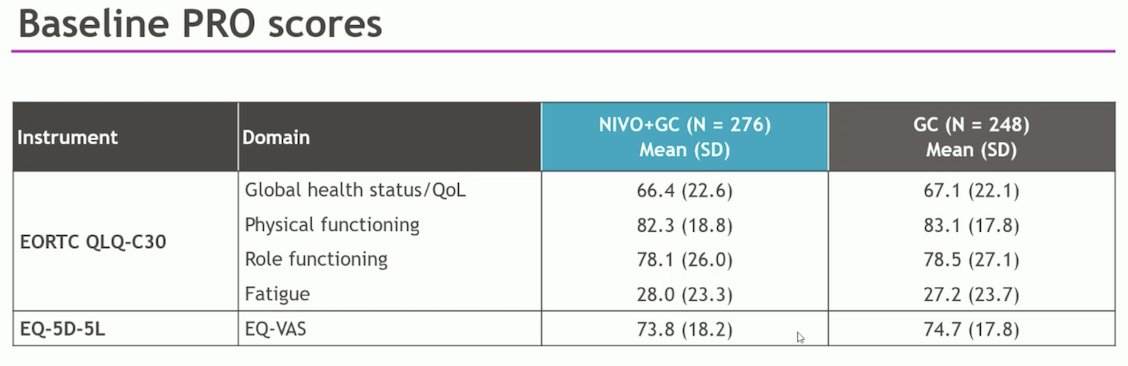
Least-squares mean changes up to week 16 showed noninferiority of Nivolumab + GC vs. GC alone on all key PRO measures, including the primary endpoint of global health status. The difference in least-squares means (95% CI) was -0.53 (-3.05 to 2.00), with the noninferiority margin set at -4.
At week 16, in terms of global health status the proportion of patients with clinically meaningful improvement (29% vs. 23%) or deterioration (28% vs 30%) Nivolumab + GC vs. GC, respectively were similar between the two arms.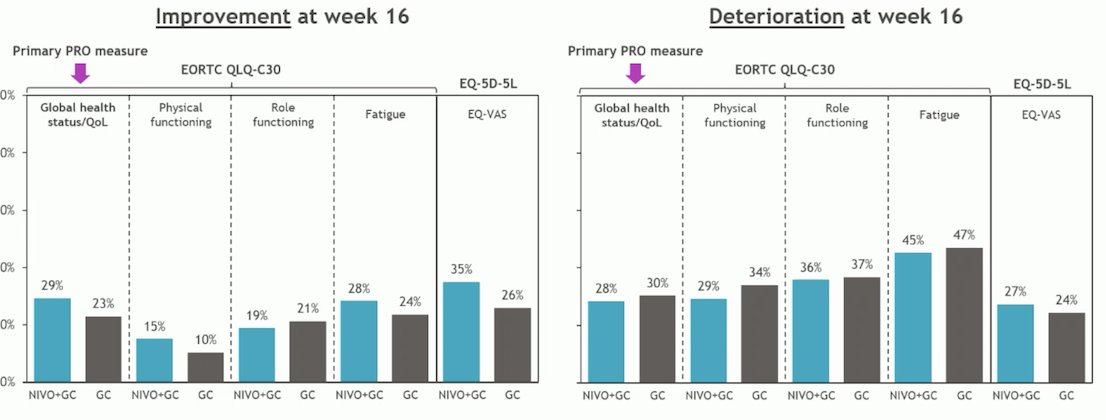
There was no clinically meaningful change over time for global health status/QoL in either arm. However, there was a trend toward improvement across visits with Nivolumab + GC as shown in the graphic below: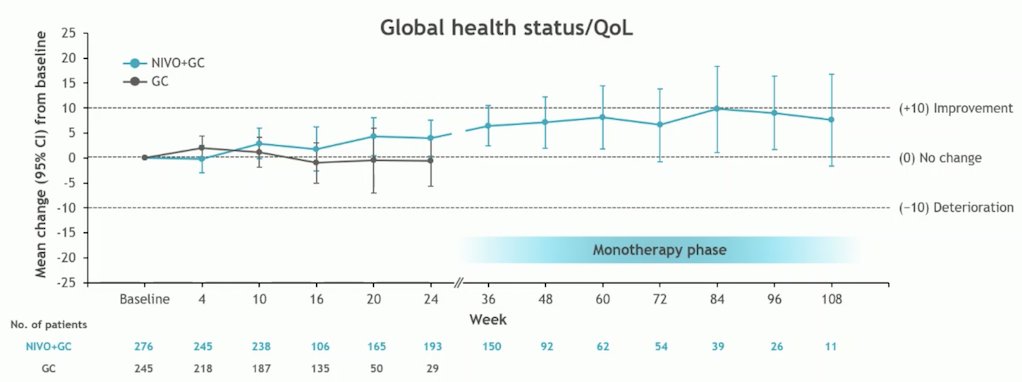
Overall, adverse events were reported in 91% of patients during the Nivolumab monotherapy phase. The most common adverse event was anemia. Treatment-related immunotherapy adverse events were more commonly endocrine-related (12.7%), with Grade 3/4 events occurring in 1.2% of patients.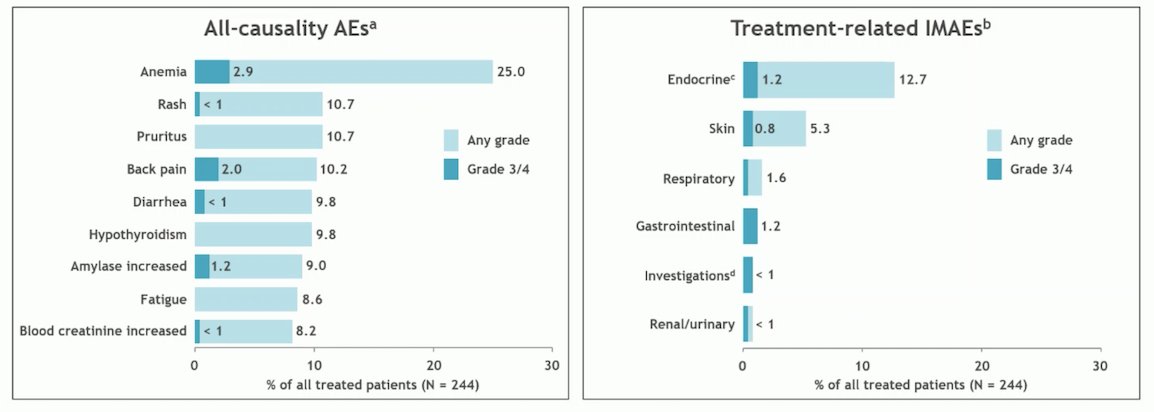
Limitations of this exploratory analysis of PRO/HRQoL in the CheckMate 901 trial were that PRO data were only available from the control arm until week 24, there was an imbalanced at week 16 between completion rates and dropped below 50% in the Nivolumab + GC arm.
Dr. Bedke summarized his presentation by stating that noninferiority was demonstrated for Nivolumab + GC vs. GC alone in the primary PRO analysis (global health status/QoL) and all other selected PRO/HRQoL measures during the combination phase. Additionally, the percentage of patients experiencing clinically meaningful changes during the combination phase was similar between the treatment arms.
CheckMate 901 showed that Nivolumab + GC (for up to 6 cycles) followed by Nivolumab monotherapy (for up to 2 years) significantly improves OS and PFS compared with GC alone in cisplatin-eligible patients with untreated unresectable or mUC while maintaining HRQoL. These findings further support this treatment strategy as a standard first-line treatment for these patients.
Presented by: Jens Bedke, MD, Professor, Urologic Oncology, Vice-Chairman of the Department of Urology, University of Tubingen, Germany.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD; CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789. doi: 10.1056/NEJMoa2309863. Epub 2023 Oct 22. PMID: 37870949.


