(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the Proffered Paper session 2: GU, non-prostate. Dr. Shilpa Gupta discussed the presentations from Drs Andromachi Kougioumtzopoulou and Jens Bedke.
Dr. Gupta began by discussing the Hellenic GU Cancer Group study exploring Nivolumab plus chemoradiotherapy in patients with non-metastatic muscle-invasive bladder cancer (MIBC) who are not undergoing cystectomy. Trimodality therapy (TMT) has not been compared head-to-head with radical cystectomy (RC). However, propensity score matching has shown that TMT is comparable to RC in patients with MIBC, with similar metastasis-free survival and distant failure-free survival.1
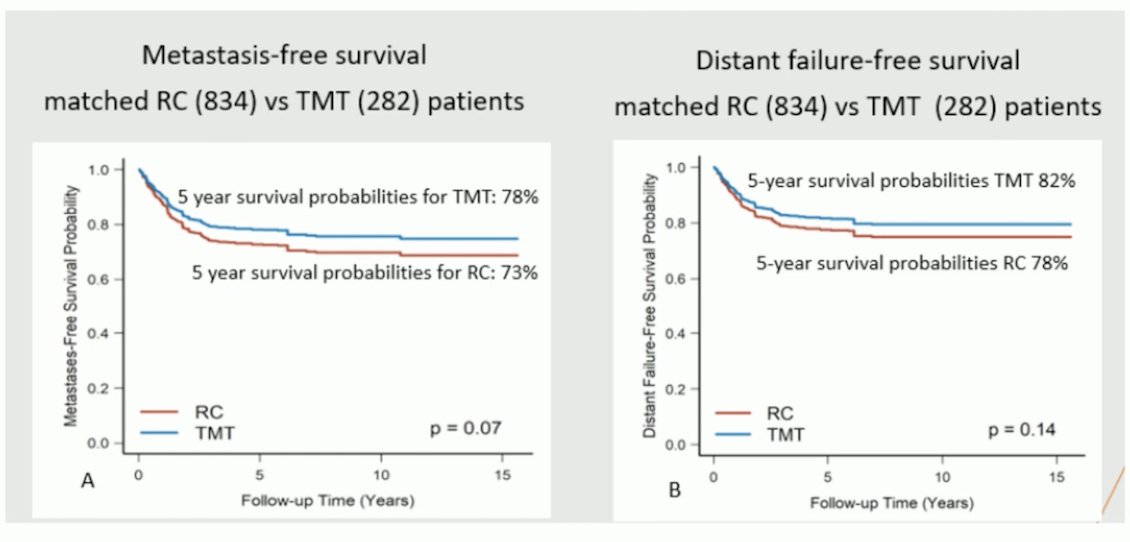
However, despite that TMT is a well-tolerated treatment and an efficacious alternative to RC in selected patients, remains vastly underutilized, and only 10-15% of eligible patients received treatment with TMT.
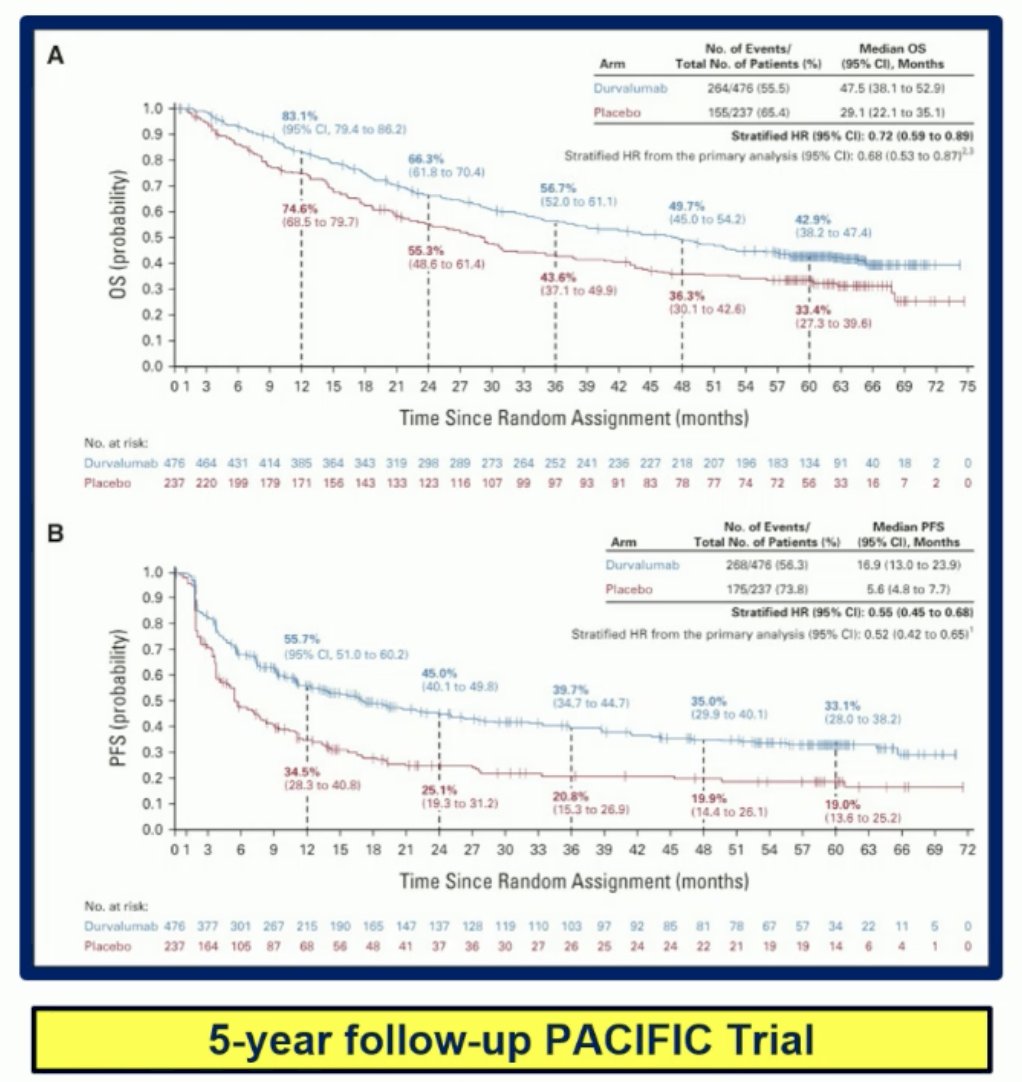
Immunotherapy and radiation therapy (RT) are synergistic across different time points. For example, in the neoadjuvant/adjuvant or perioperative setting, this synergy has been demonstrated. The PACIFIC trial, which explored durvalumab after chemoradiotherapy (CRT) for stage III non-small-cell lung cancer, has shown significant improvements in overall survival (OS) and progression-free survival (PFS).2
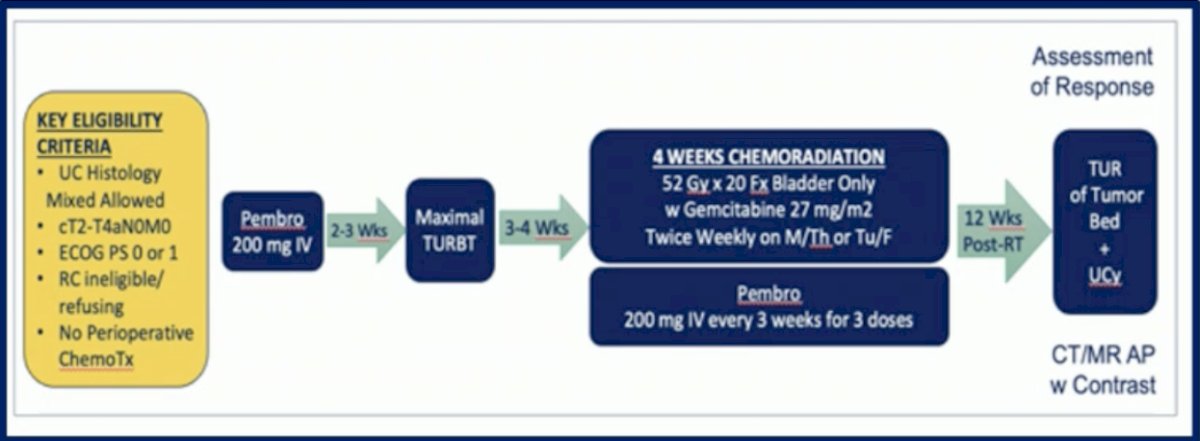
The data with IO and RT in MIBC comes from a phase II small study that enrolled 54 patients with urothelial cancer cT2-T4aN0M0 who were ineligible or refused RC, patients received Pembrolizumab followed by maximal TURBT followed by 4-weeks of CRT and Pembrolizumab. The two year bladder intact disease-free survival was 70%, unfortunately, it did not meet the pre-defined success threshold of 80%. A total of 11% of patients required salvage RC and there were 22% local and distant recurrences.3
Similarly in a phase I study of concurrent Atezolizumab and hypofractioned RT and Gemcitabine were associated with unacceptable toxicity which led to the early closure of the study.
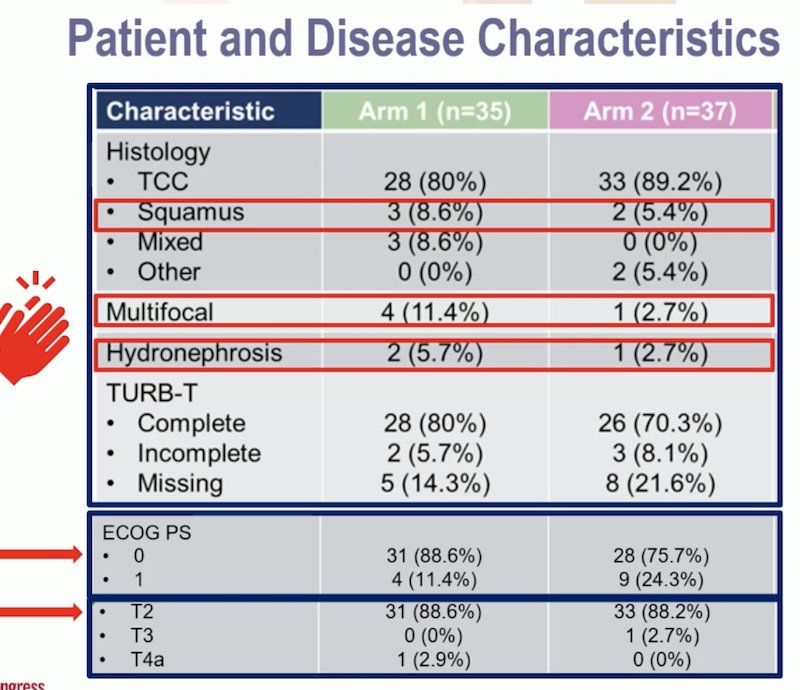
Dr. Gupta reviewed the Hellenic GU Cancer Group study design, congratulating the investigators for including patients with variant histology. She noted that the CRT was using only cisplatin, and they employed a "sandwich therapy" approach in the Nivolumab + CRT arm, administering Nivolumab before, concurrent, and after RT started. Additionally, the study used a GFR threshold of 40 mL/min, which is lower than the traditional 60 mL/min.

The study also included only ECOG PS 0-1 patients and permitted those with hydronephrosis, which has historically been considered a contraindication for TMT. The majority of the patients were ECOG 0 and had T2 disease.
In the combination arm, only 64.9% of patients completed chemotherapy compared to 91.4% in the control arm. Additionally, only 78% of patients completed Nivolumab. We need to investigate the causes behind these lower completion rates for chemotherapy and immunotherapy by examining the adverse event profile of the combination arm.
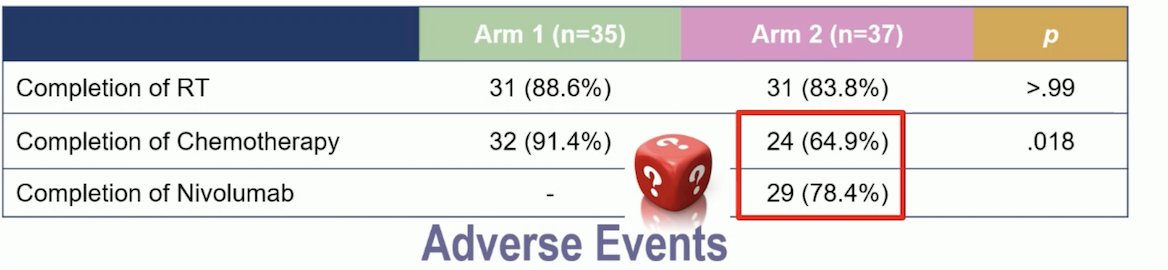
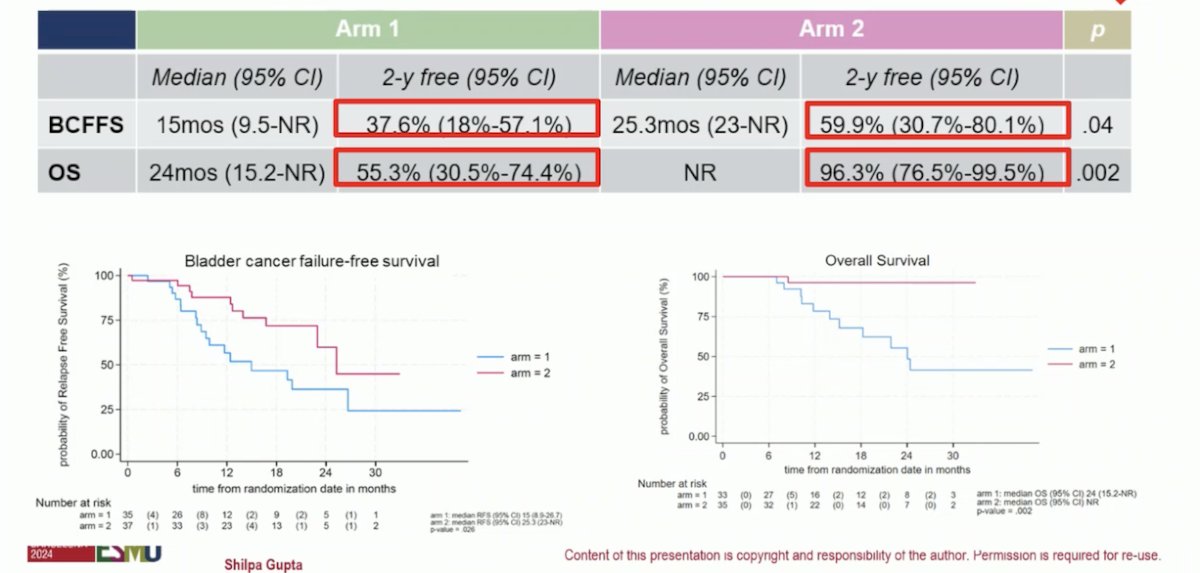
The addition of Nivolumab to TMT improved bladder cancer failure-free survival and OS as illustrated below. Distant relapses were only 2,6% with the combination compared to 28.2% in the control arm. The primary endpoint of this trial (2-year locoregional relapse-free-survival) has not been evaluated yet.
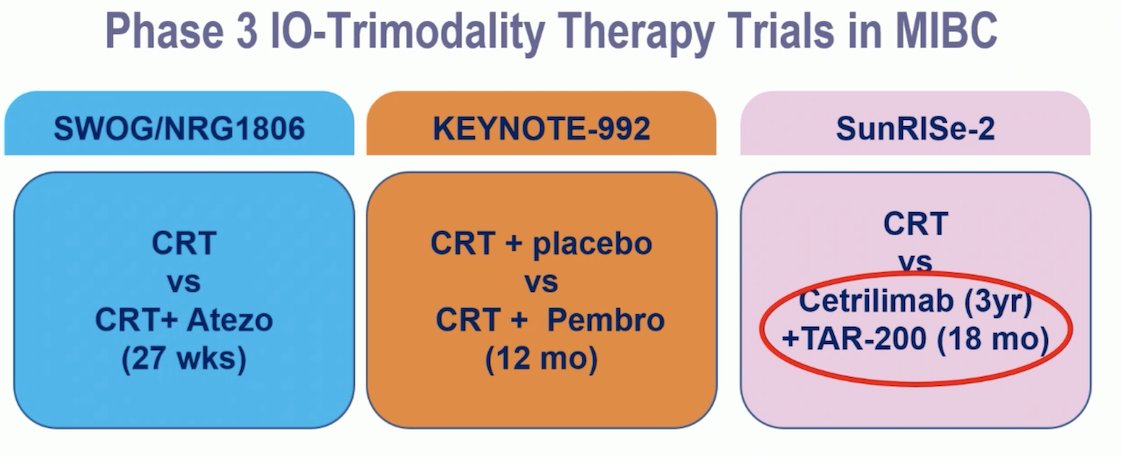
Dr. Gupta presented three Phase III TMT trials in MIBC:
- The SWOG/NRG 1806 trial, which compares TMT versus TMT + Atezolizumab, has completed accrual.
- The Keynote-992 trial, exploring TMT + Placebo versus TMT + Pembrolizumab, is expected to complete accrual this month.
- The SunRISe-2 trial is evaluating TMT versus Cetrilimab for 3 years plus TAR-200 for 1.5 years.
There are inconsistencies in the designs of these ongoing trials, and the duration of IO therapy varies significantly across them.
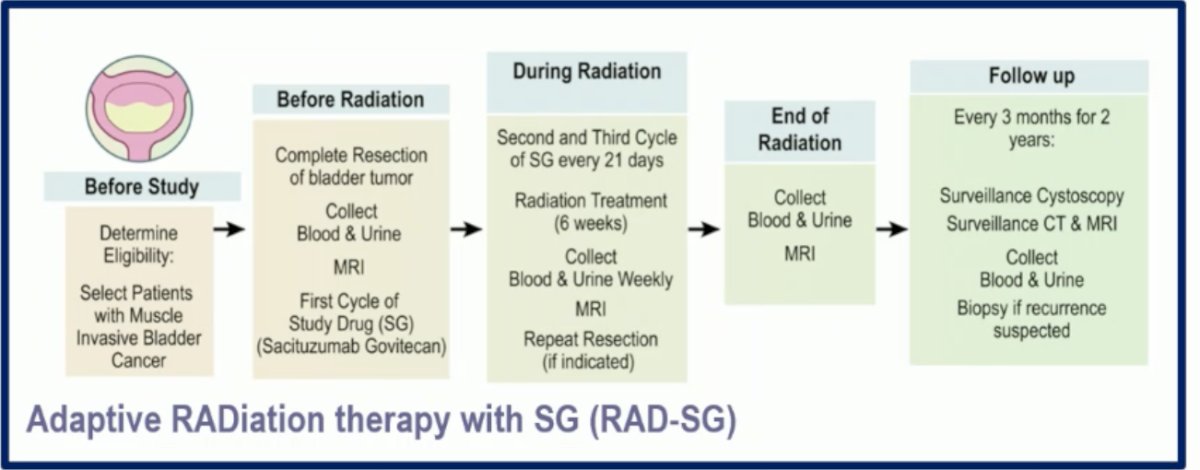
We have three antibody-drug conjugates (ADCs) in bladder cancer: Sacituzumab govitecan (SG), targeting Trop-2; Enfortumab vedotin (EV), targeting Nectin-4; and Trastuzumab Deruxtecan (T-DXd), targeting HER-2. All these ADCs have shown significant activity in the metastatic bladder cancer setting. The combination of ADCs with immuno-oncology (IO) agents, especially EV + Pembrolizumab, offers a unique opportunity to potentially combine with radiation therapy (RT) or even avoid it. Dr. Gupta discussed the RAD-SG trial, which is exploring the combination of SG with RT in selected patients with MIBC through an adaptive radiation therapy approach.
The RETAIN trial (NCT02710734) is a single-arm, Phase II, non-inferiority trial investigating risk-adapted therapy following neoadjuvant chemotherapy for MIBC. It enrolled patients with cT2-T3N0M0 urothelial carcinoma who received NAC with accelerated MVAC. Pre-NAC TURBT specimens were sequenced for mutations in ATM, ERCC2, FANCC, or RB1. Patients with at least one mutation and no clinical evidence of disease by restaging TURBT, urine cytology, and imaging after NAC began predefined active surveillance (AS). Those without these criteria underwent bladder-directed therapy, either TMT or cystectomy. The trial did not meet its 2-year metastasis-free survival (MFS) endpoint, and investigators are now working on the RETAIN-2 trial, which incorporates three cycles of Nivolumab with accelerated MVAC chemotherapy.4
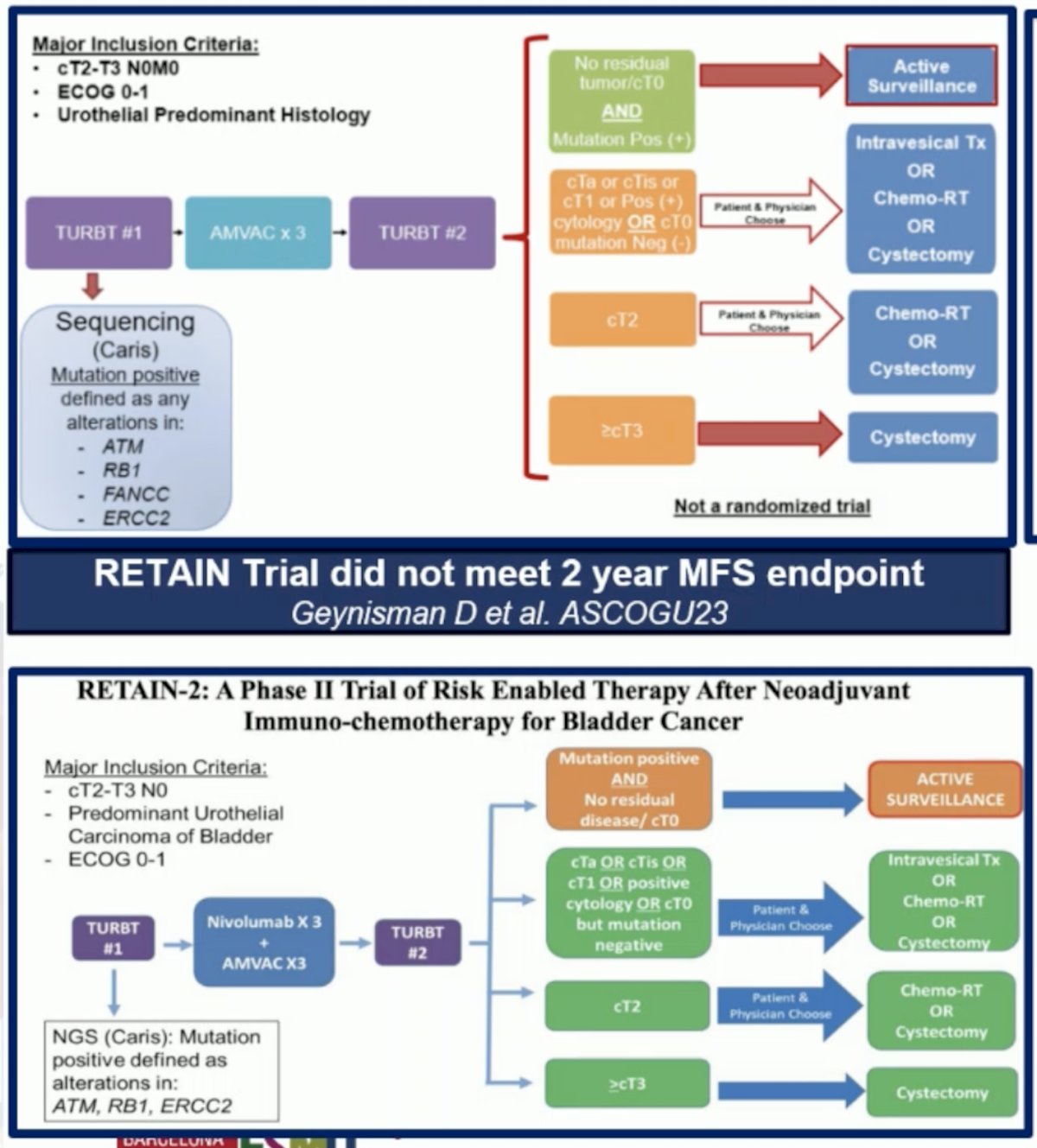
Dr. Galsky discussed the HCRN-GU16-257 trial, which investigated GC + Nivolumab for four cycles, followed by re-staging and randomization to either radical cystectomy (RC) or no RC based on clinical complete response (cCR). Of the patients who achieved cCR (43%), 33 were given the option to undergo RC or not; 32 chose not to have RC and were instead offered an additional four months of Nivolumab. Of these 32 patients, 8 eventually underwent cystectomy due to local recurrence. The trial successfully met its co-primary endpoint, demonstrating that cCR is a predictive marker for treatment benefit.5
Finally, Dr. Galsky briefly discussed the Alliance study (A031701), a multicenter Phase II trial enrolling 271 cisplatin-eligible patients with T2-T4aN0/xM0 disease. Participants will receive either standard dose or dose-dense gemcitabine and cisplatin chemotherapy, with simultaneous genetic sequencing of pre-treatment TURBT specimens. Patients whose tumors harbor deleterious alterations in any one of nine pre-selected DDR genes (ERCC2, ERCC5, BRCA1, BRCA2, RECQL4, RAD51C, ATM, ATR, and FANCC) and who show a <T1 response on clinical restaging are eligible for TMT. Patients without deleterious DDR gene alterations or with >T1 disease after NAC will proceed to RC or TMT. This trial is currently ongoing.6
Dr. Gupta concluded that Nivolumab + TMT demonstrates encouraging preliminary efficacy and safety, but we await primary endpoint and follow-up data. There is a need for adaptive trials with clearly defined endpoints (such as BIDFS, LCR, cCR) and precise patient selection criteria. The incorporation of mpMRI, immune/gene signatures, cDNA, and AI could help predict which patients will benefit from TMT or IO + TMT combination approaches. For future TMT trials, it is essential to harmonize the timing, sequencing, and duration of systemic therapy, the type and dose of RT, and to further investigate long-term toxicity and patient-reported outcomes (PRO) assessments in TMT.
Health-related quality of life from CheckMate 901 trialSeveral factors influence a cancer patient’s health-related quality of life (HRQoL), including patient-related, disease-related, treatment-related, psychological, and socioeconomic, cultural, and environmental factors. The goal of cancer therapy should be to extend survival while maintaining or improving HRQoL. Patient-reported outcomes (PROs) are crucial in clinical trials for evaluating patient-centered outcomes.
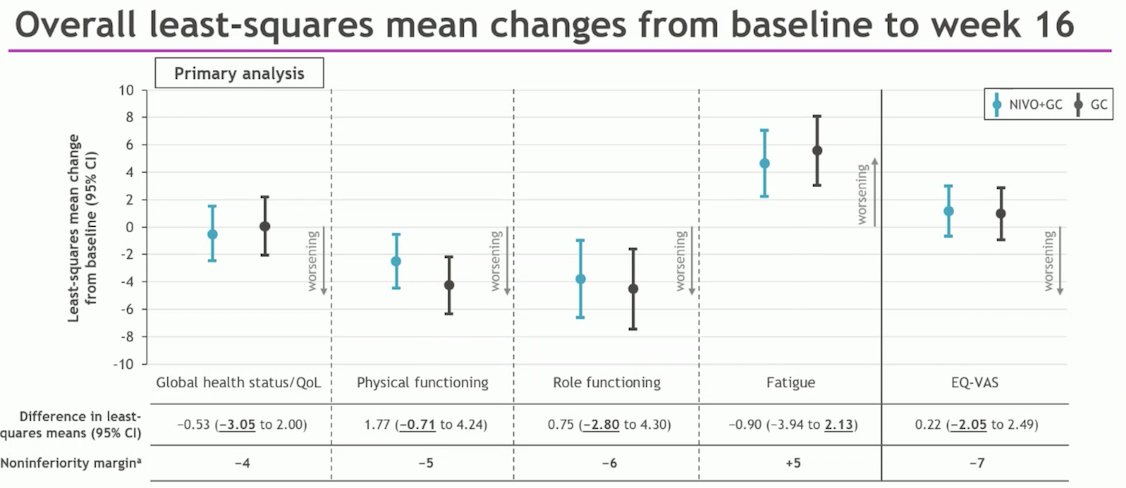
The CheckMate 901 trial, which achieved positive results for its primary endpoints of overall survival (OS) and progression-free survival (PFS), included an exploratory analysis of PROs. This analysis assessed changes from baseline to week 16 in global health status during both the combination phase and the nivolumab monotherapy phase. The EORTC QLQ-C30 was used to evaluate global health status.
The results demonstrated noninferiority of GC + Nivolumab compared to GC across all PRO measures through week 16, with no clinically meaningful worsening or improvement associated with the combination therapy. The results of the study are shown below:
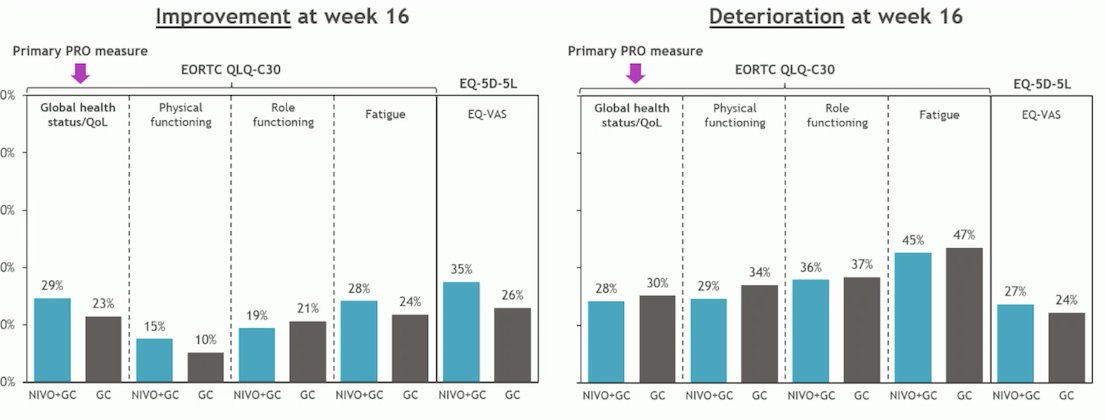
Dr. Gupta compared this data with PRO results from other bladder cancer trials involving immuno-oncology (IO) agents. The Keynote 045 trial was the first IO trial to explore PROs and found that HRQoL was better with Pembrolizumab compared to salvage chemotherapy.7 The Imvigor 130 trial, which investigated first-line chemotherapy + Atezolizumab versus placebo with platinum-based chemotherapy, showed a trend towards improved time to deterioration in HRQoL compared to chemotherapy alone.8 In contrast, the CheckMate 274 trial, which compared adjuvant Nivolumab versus placebo for high-risk MIBC, found no significant difference in HRQoL between Nivolumab and placebo.9 Similarly, the JAVELIN Bladder 100 trial also reported no difference in HRQoL between avelumab and placebo.

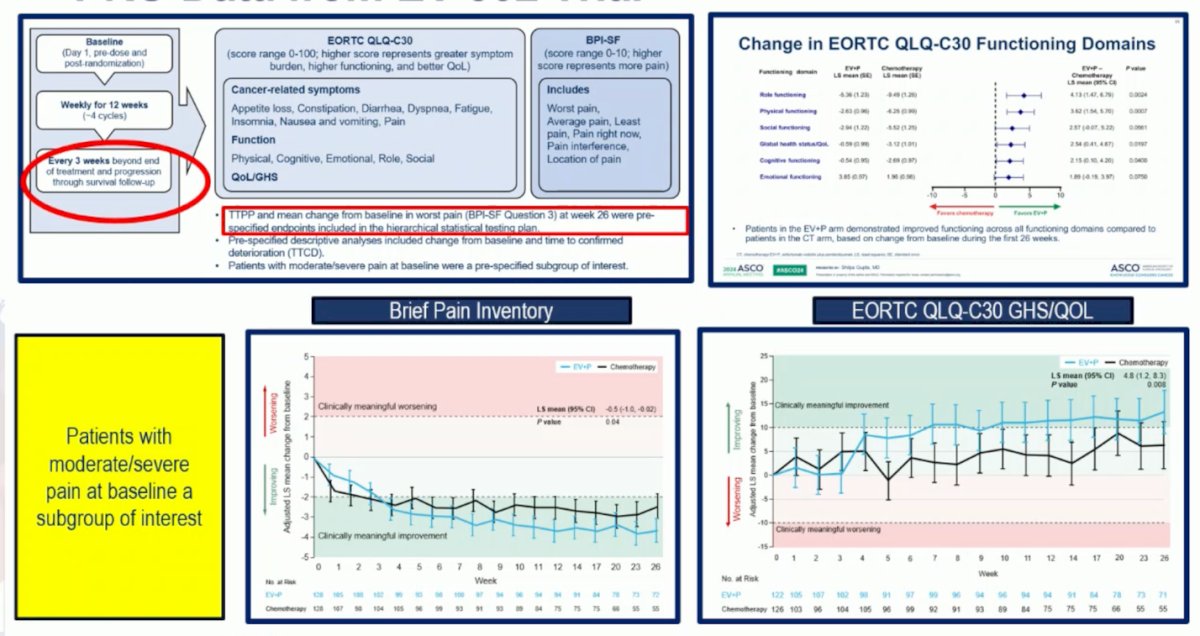
In the EV-302 trial, the PRO assessment continued throughout the journey, even after progression, and when receiving subsequent therapies, time-to pain progression and time to worse pain were pre-specified endpoints of the trial. Patients with moderate to severe pain at baseline who were treated with EV+P had a meaningful improvement from baseline in BPI worst pain and Global Health Status/Quality of Life.10
Unfortunately, HRQoL tools have not evolved as quickly as advances in cancer therapy. Historically, assessment instruments have used the same questions regardless of disease type, stage, or type of therapy (chemotherapy or IO). Commonly used tools often fail to differentiate between disease symptoms, physical function, and symptomatic adverse events. Tools like the NCI PRO-CTCAE, FACIT-GP-5, and QTWIST are more effective for assessing symptoms related to treatment.
Dr. Gupta concluded her critical appraisal of this exploratory analysis of CheckMate 901 PRO/HRQoL by saying:
- This analysis showed noninferiority for Nivolumab + GC compared to GC alone at week 16 during the combination phase
- There is a dire need for contemporary tools to assess PROs in the novel therapeutic era to capture the unique toxicities
- We need to harmonize PRO assessment in trials, move away from exploratory endpoints, and make these primary endpoints
- Selection of symptomatic patients at baseline to understand the impact of treatment on HRQoL and pain
- Important to consider PRO assessment throughout a patient's journey and not just during active treatment (EV-302)
Presented by: Shilpa Gupta, MD, Director, Genitourinary Medical Oncology, Taussig Cancer Institute, Co-Leader of the Genitourinary Oncology Program, Cleveland Clinic, Cleveland, OH
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, Drumm M, Mari A, Thio E, Fleshner NE, Kulkarni GS, Jewett MAS, Bristow RG, Catton C, Berlin A, Sridhar SS, Schuckman A, Feldman AS, Wszolek M, Dahl DM, Lee RJ, Saylor PJ, Michaelson MD, Miyamoto DT, Zietman A, Shipley W, Chung P, Daneshmand S, Efstathiou JA. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6):669-681. doi: 10.1016/S1470-2045(23)00170-5. Epub 2023 May 12. PMID: 37187202.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017 Nov 16;377(20):1919-1929. doi: 10.1056/NEJMoa1709937. Epub 2017 Sep 8. PMID: 28885881.
- Arjun Vasant Balar et al. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): A multicenter phase 2 trial.. JCO 39, 4504-4504(2021).DOI:10.1200/JCO.2021.39.15_suppl.4504
- Daniel M. Geynisman et al. A phase II trial of risk-enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN).. JCO 41, 438-438(2023). DOI:10.1200/JCO.2023.41.6_suppl.438
- Galsky, M.D., Daneshmand, S., Izadmehr, S. et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat Med 29, 2825–2834 (2023). https://doi.org/10.1038/s41591-023-02568-1
- Gopa Iyer et al. A phase II study of gemcitabine plus cisplatin chemotherapy in patients with muscle-invasive bladder cancer with bladder preservation for those patients whose tumors harbor deleterious DNA damage response (DDR) gene alterations (Alliance A031701).. JCO 40, TPS4615-TPS4615(2022) DOI:10.1200/JCO.2022.40.16_suppl.TPS4615
- David J. Vaughn et al. Health-Related Quality-of-Life Analysis From KEYNOTE-045: A Phase III Study of Pembrolizumab Versus Chemotherapy for Previously Treated Advanced Urothelial Cancer. JCO 36, 1579-1587(2018).DOI:10.1200/JCO.2017.76.9562
- Bamias, A. et al. 698O Patient-reported outcomes (PROs) from IMvigor130: A global, randomised, partially blinded phase III study of atezolizumab (atezo) + platinum-based chemotherapy (PBC) vs placebo (PBO) + PBC in previously untreated locally advanced or metastatic urothelial carcinoma (mUC). Annals of Oncology, Volume 31, S551 - S552
- Witjes, Johannes Alfred et al. Health-related Quality of Life with Adjuvant Nivolumab After Radical Resection for High-risk Muscle-invasive Urothelial Carcinoma: Results from the Phase 3 CheckMate 274 Trial. European Urology Oncology, Volume 5, Issue 5, 553 - 563
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.


