(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a proffered paper session for non-prostate genitourinary malignancies. Professor Andrea Necchi presented the results of an interim analysis of SunRISe-4, a randomized phase II trial of TAR-200 plus cetrelimab versus cetrelimab alone as neoadjuvant therapy in patients with muscle-invasive bladder cancer (MIBC) who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy.
A standard of care approach for MIBC (cT2-T4aN0M0) includes radical cystectomy with or without neoadjuvant chemotherapy (NAC).1 However, up to 50% of patients with MIBC are ineligible for NAC.2,3 Approximately 50% of patients experience recurrence within two years of radical cystectomy, and the 5-year survival after radical cystectomy is ~ 50%.4-6
In patients with MIBC undergoing a radical cystectomy, pathologic stage is a prognostic factor for survival.5-8 Pathologic complete response (pCR) rates with radical cystectomy alone, with NAC, and with neoadjuvant checkpoint inhibitors are 10–15%, ~40%, and 31–39%, respectively.7-13 A pCR in patients who have received NAC is associated with a 55% lower risk of death and an 81% lower risk of recurrence compared to patients with residual disease.8 There is a high unmet need for effective and more tolerable treatment options for patients with MIBC who are candidates for radical cystectomy, but not candidates for or who refuse NAC.
TAR-200 is a gemcitabine intravesical releasing system designed to provide sustained gemcitabine within the bladder. Phase I studies have shown clinical activity for TAR-200 in MIBC patients. 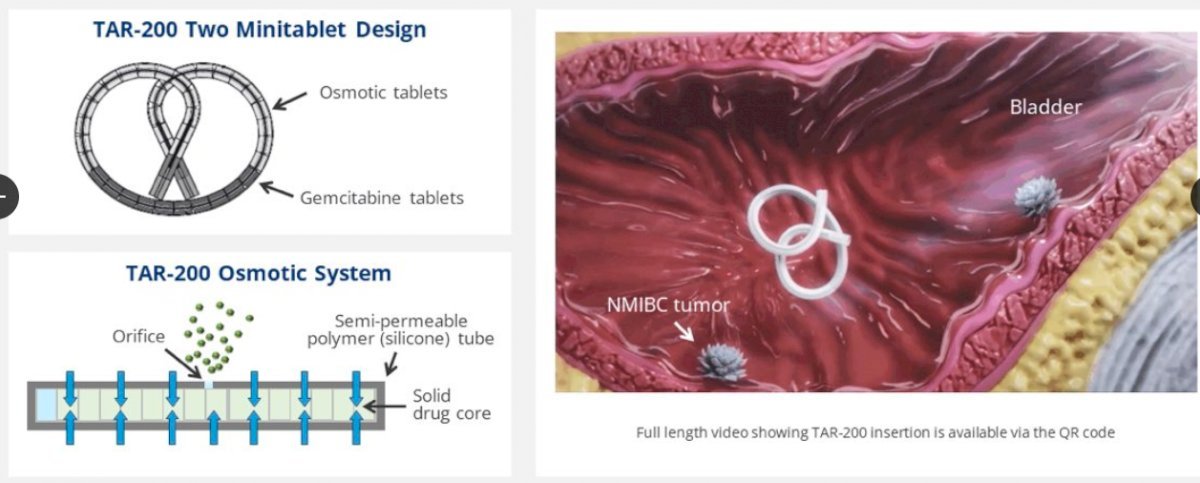
Cetrelimab is an anti-PD-1 agent. SunRISe-4 (NCT04919512) is an ongoing randomized phase II study assessing the efficacy and safety of neoadjuvant TAR-200 + cetrelimab or cetrelimab monotherapy in patients with MIBC scheduled for radical cystectomy and who are ineligible for or refuse NAC.
The study design is summarized below. The key trial eligibility criteria were as follows:
- cT2-4aN0M0 MIBC
- Predominant urothelial carcinoma histology
- Ineligible for or refusing NAC
ECOG performance status 0–1
Patients underwent 5:3 randomization to:
- TAR-200 225 mg gemcitabine every 3 weeks (indwelling) for 12 weeks + cetrelimab intravenously for 12 weeks (4 cycles)
- Cetrelimab intravenously for 12 weeks (4 cycles)
Randomization was stratified by the absence or presence of visible residual disease at TURBT and tumor stage at MIBC diagnosis (cT2 versus cT3-4a). The planned sample size was n=160. The primary endpoint was a pathologic complete response. Key secondary endpoints were recurrence-free survival, safety, pathologic objective response (pOR; i.e., ≤ypT1N0), and overall survival. For this interim analysis, the clinical data cutoff was May 31, 2024.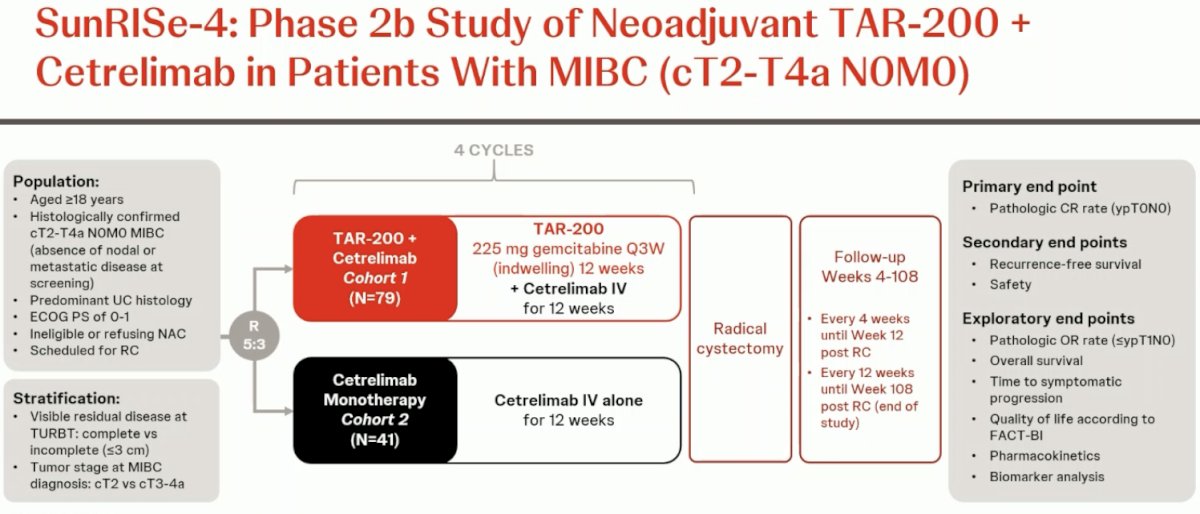
The statistical plan is summarized below: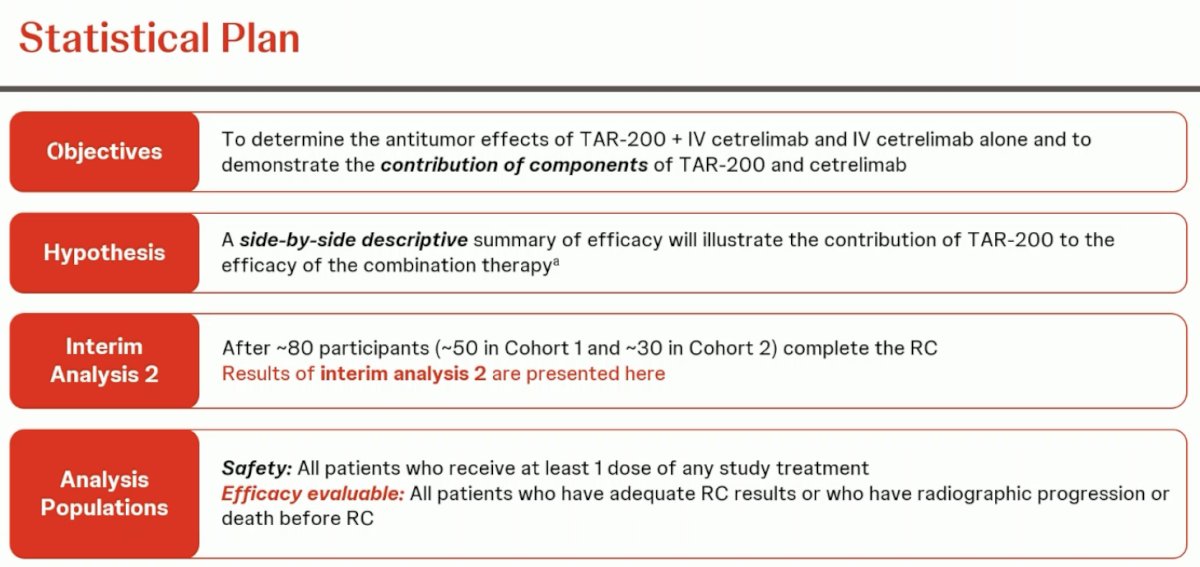
Dr. Necchi highlighted that this is not a comparative trial. The goal is to describe the efficacy outcomes in each arm without directly comparing them. The interim analysis for this report was presented after the first 80 participants completed their radical cystectomy. The efficacy evaluable patients for this report were those who had adequate radical cystectomy results or who had radiographic progression or death prior to radical cystectomy.
A total of 122 patients were randomized, 80 to Cohort 1 of TAR-200 + cetrelimab and 42 to Cohort 2 of cetrelimab monotherapy. The efficacy evaluable population included 53 and 31 patients from Cohorts 1 and 2, respectively.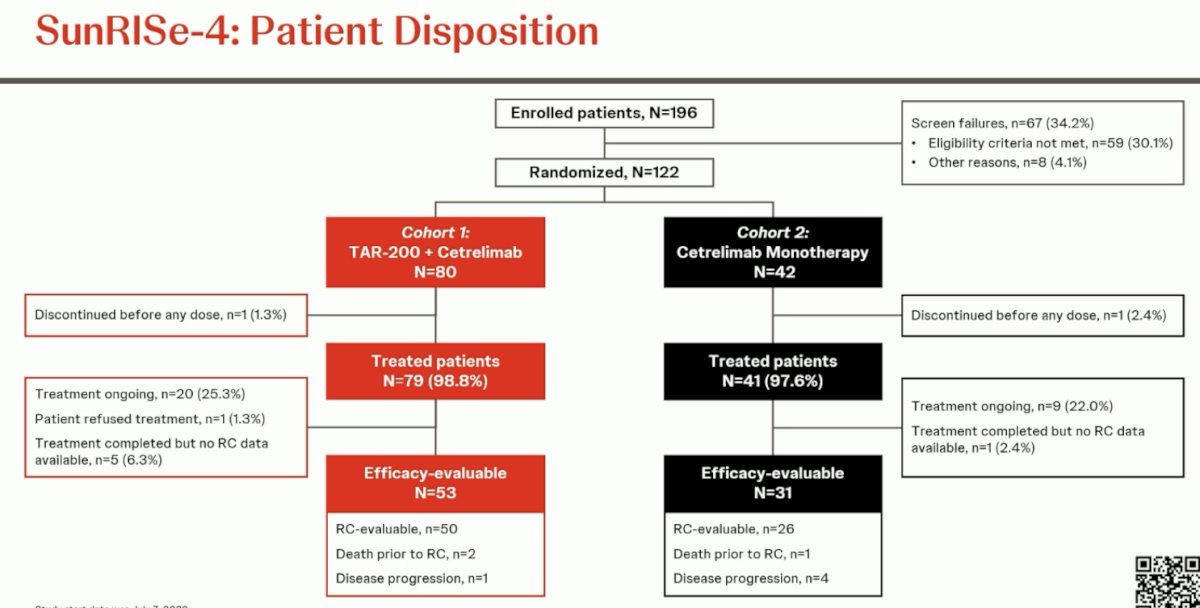
The baseline patient characteristics are summarized below. Cisplatin ineligibility was present in 37–39% of patients, and 15–20% had residual disease on TURBT. 79–85% of patients had cT2 disease at diagnosis. Variant histology was present in 20–27% of tumors. 13–20% had received prior intravesical therapy.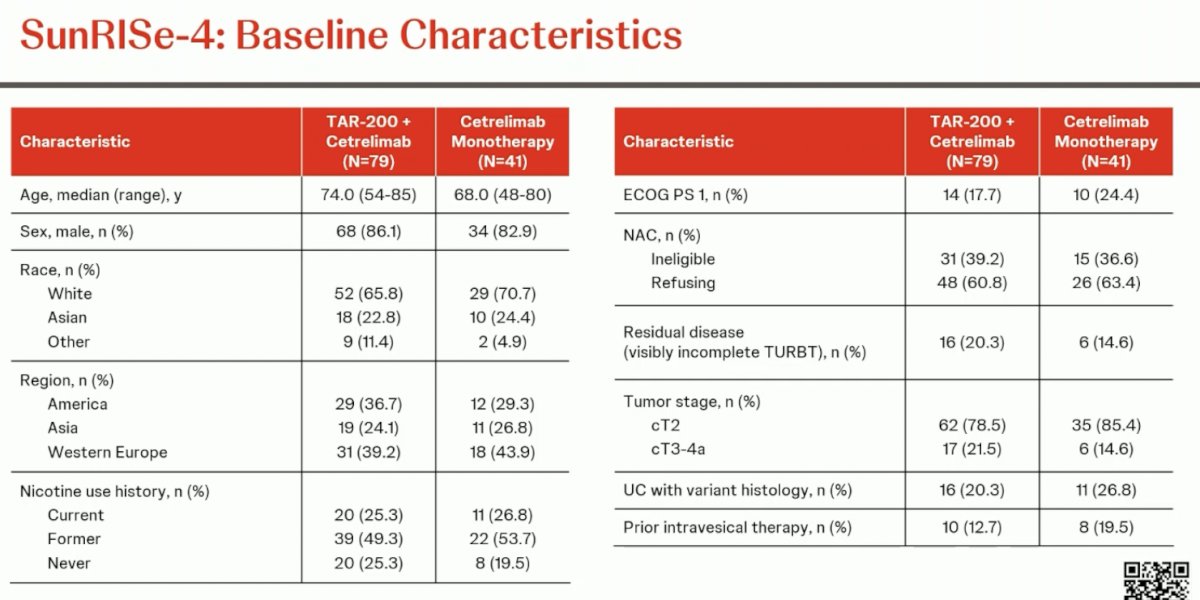
The pCR in the combination arm was 42%, and the pathologic objective response (pCR + ≤ypT1N0) was 60%. The corresponding proportions in the cetrelimab monotherapy arm were 23% and 36%, respectively.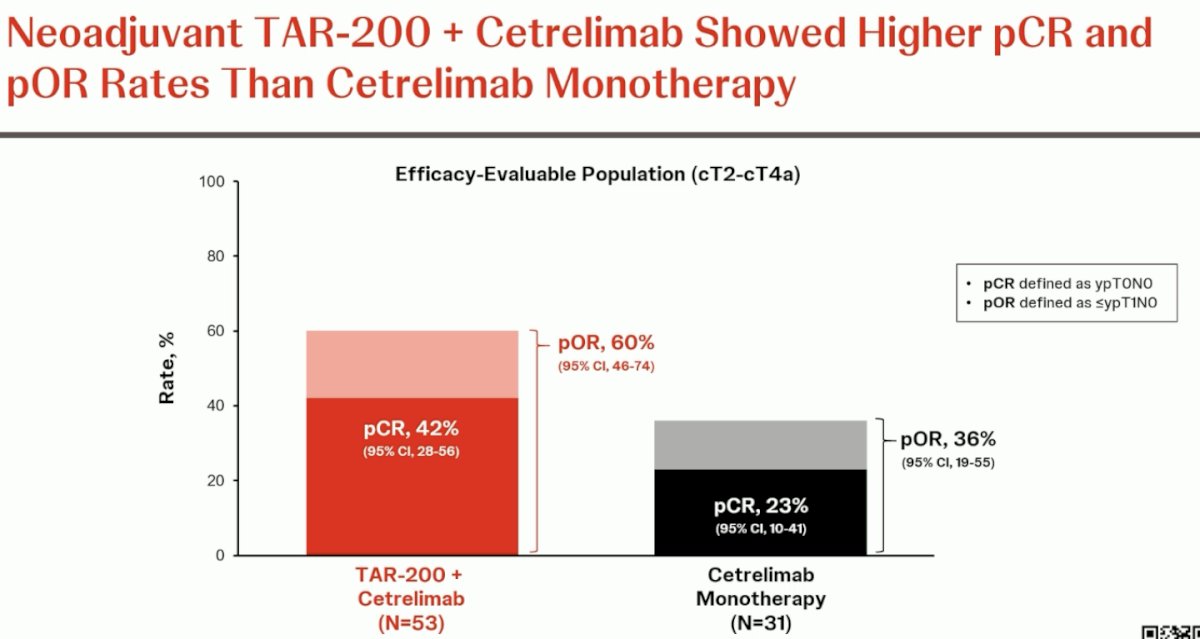
Next, these outcomes were assessed, stratified by clinical stage, and completeness of TURBT. In cT2 patients, the pCR and pOR rates were 48% and 68% respectively. Conversely, in cT3-4a patients, the corresponding rates were lower at 23% and 39%, respectively. Patients with an incomplete TURBT (n=9 only) had pCR and pOR rates of 56% and 67%, respectively. Those with a complete TURBT had rates of 39% and 59%, respectively.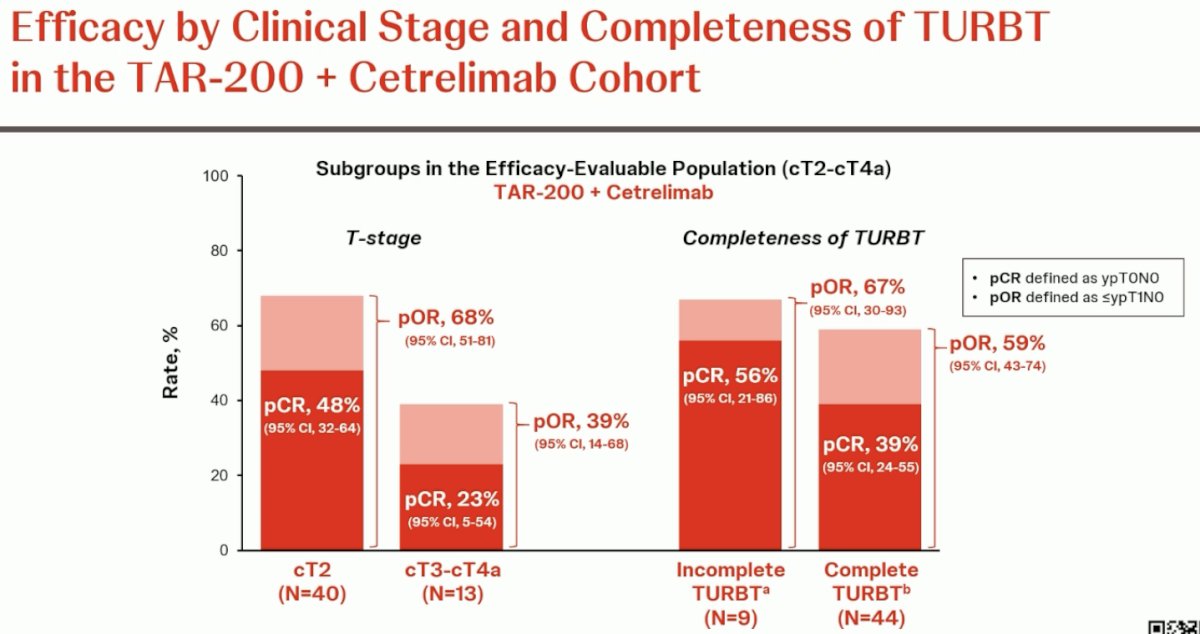
The corresponding rates by clinical stage and TURBT completeness in the cetrelimab monotherapy arm are summarized below.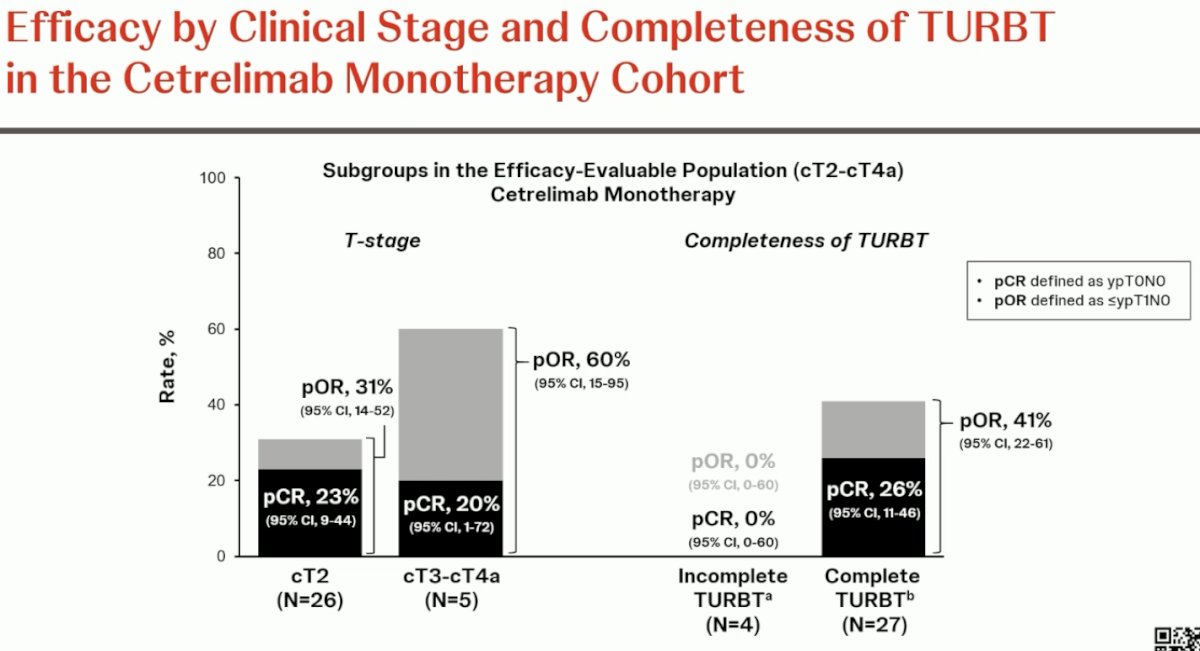
The efficacy of TAR-200 improved with an increasing number of doses administered. The pCR proportion increased from 27% to 30% to 50% in patients who received 1–2, 3, and the full 4 doses, respectively.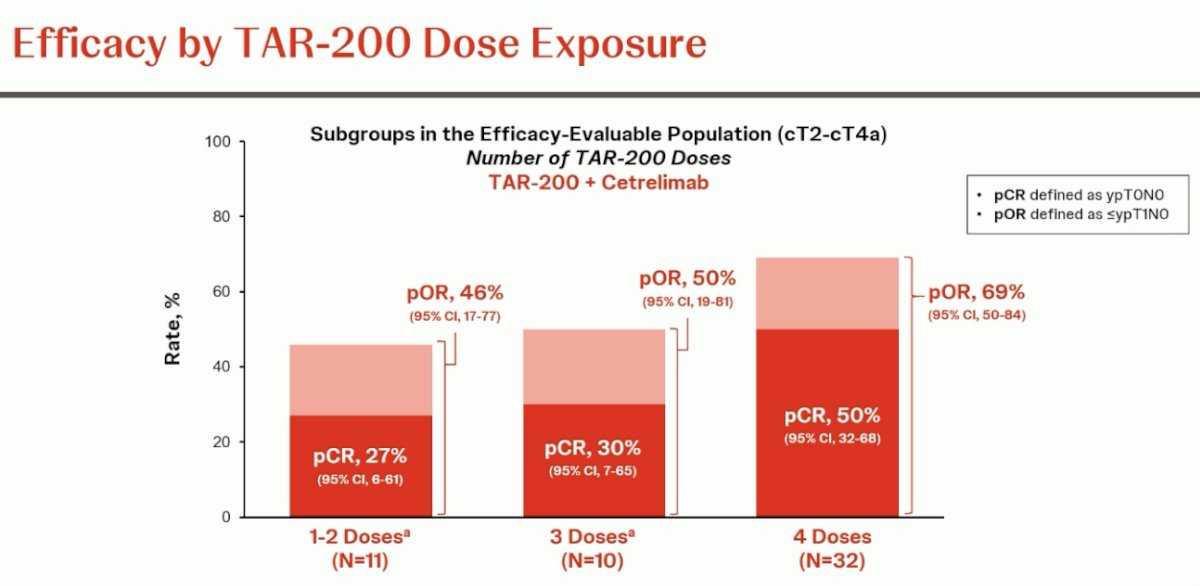
Immune-related grade ≥3 adverse events were observed in 6.3% of patients in the TAR-200 + cetrelimab arm and 5% of patients in the cetrelimab monotherapy arm. Overall, grade ≥3 treatment-related adverse events were observed in 11.4% and 5% of patients in the combination and monotherapy arms, respectively. Adverse events leading to treatment discontinuation were observed in 13% of patients in the combination arm and none in the monotherapy arm. The median time to radical cystectomy was 13.7 weeks in the combination arm and 12.6 weeks in the monotherapy arm.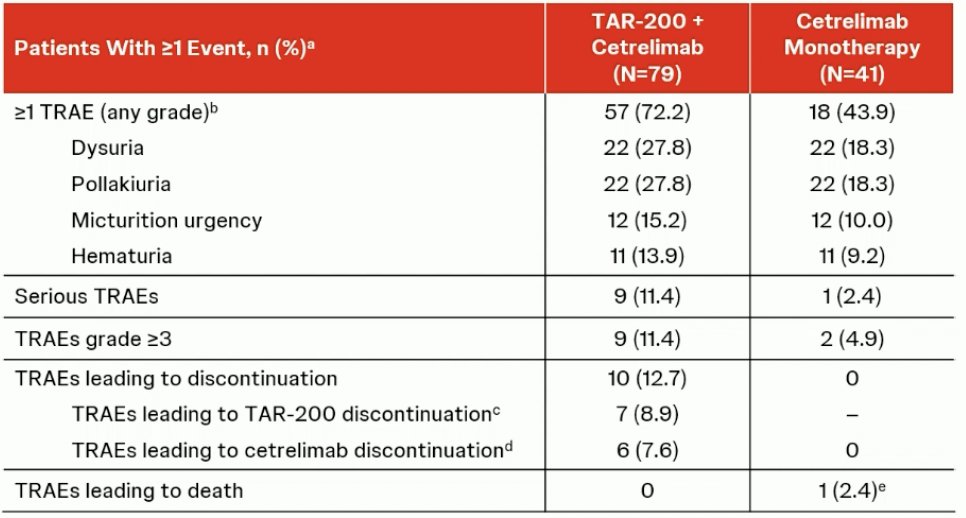
Dr. Necchi concluded as follows:
- The combination of neoadjuvant TAR-200 + cetrelimab showed pCR and pOR rates of 42% and 60%, respectively, in patients with MIBC
- In the cT2 subgroup, 48% of patients treated with TAR-200 + cetrelimab achieved pCR, and 68% were downstaged to ≤pT1 at radical cystectomy
- Cetrelimab monotherapy provided pCR and pOR rates of 23% and 35%, respectively.
- TAR-200 + cetrelimab had a manageable safety profile in the neoadjuvant setting
- Most treatment-related adverse events with TAR-200 + cetrelimab were low-grade
- The rate of discontinuations due to treatment-related adverse events was low at 13%
- SunRISe-4 demonstrates for the first time a benefit of the addition of TAR-200, an intravesical targeted releasing system, to checkpoint inhibition as neoadjuvant treatment in patients with MIBC.
Presented by: Professor Andrea Necchi, MD, Director of GU Medical Oncology, San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: SunRISe-4 Trial Explores TAR-200 and Cetrelimab Combination for Bladder Cancer Treatment - Andrea Necchi
References:
- Scarpato KR, Morgans AK, Moses KA. Optimal management of muscle-invasive bladder cancer - a review. Res Rep Urol. 2015; 7:143-51.
- Thompson RH, Boorjian SA, Kim SP, et al. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 2014; 113(5b):E17-21.
- Jiang DM, Gupta S, Kitchlu A, et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat Rev Urol. 2021; 18(2):104–14.
- Li G, Niu HM, Wu HT, et al. Effect of cisplatin-based neoadjuvant chemotherapy on survival in patients with bladder cancer: a meta-analysis. Clin Invest Med. 2017; 40(2): E81-94.
- Stein JP, Lieskovsky G, Cote R, et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J Clin Oncol. 2023; 41(22):3772-81.
- Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003; 21:690-6.
- Isbarn H, Karakiewicz PI, Shariat SF, et al. Residual pathological stage at radical cystectomy significantly impacts outcomes for initial T2N0 bladder cancer. J Urol. 2009; 182(2):459-65.
- Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014; 65(2):350-7.
- Bandini M, Gibb EA, Gallina A, et al. Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study☆. Ann Oncol. 2020; 31(12): 1755-63.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019; 25(11):1706-14.
- Szabados B, Kockx M, Assaf ZJ, et al. Final Results of Neoadjuvant Atezolizumab in Cisplatin-ineligible Patients with Muscle-invasive Urothelial Cancer of the Bladder. Eur Urol. 2022; 82(2):212-22.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003; 349(9):859-66.
- Necchi A, Faltas BM, Slovin SF, et al. Immunotherapy in the Treatment of Localized Genitourinary Cancers. JAMA Oncol. 2023; 9(10):1447-54.
Related Content:
SunRISe-4 Trial Explores TAR-200 and Cetrelimab Combination for Bladder Cancer Treatment - Andrea Necchi
ESMO 2024: Invited Discussant: JCOG1019, TOMBOLA, and SunRISe-4


