(UroToday.com) The 2024 ESMO annual meeting included a session on kidney cancer, featuring a presentation by Dr. Toni Choueiri discussing results of the phase III TiNivo-2 trial assessing tivozanib + nivolumab versus tivozanib monotherapy in patients with RCC following 1 or 2 prior therapies including an immune checkpoint inhibitor. Immune checkpoint inhibitors are the cornerstone of the first line treatment of advanced metastatic RCC, however the optimal sequence in patients whose disease has progressed after treatment with immune checkpoint inhibitors is uncertain, leaving several unanswered questions:
- Can immune checkpoint inhibitor rechallenge improve clinical outcomes?
- Can outcomes be impacted if non-immune checkpoint inhibitors were used before immune checkpoint inhibitor rechallenge? (ie. immune checkpoint inhibitor break)?
- Are there any differences between anti-PD-1 or anti-PD-L1 therapies in the rechallenge setting?
Evidence supports the value of VEGFR TKI use, including tivozanib in patients previously treated with immune checkpoint inhibitor regimens. Previously, tivozanib has demonstrated single agent activity following VEGFR TKI and immune checkpoint inhibitor, and in combination with immune checkpoint inhibitor. As such, TiNivo-2 was designed to test the combination of the PD-1 inhibitor nivolumab and tivozanib versus tivozanib monotherapy following tumor progression on prior immune checkpoint inhibitor-based therapy.
TiNivo-2 enrolled patients with advanced clear-cell RCC who had 1–2 prior lines of therapy, including an immune checkpoint inhibitor. Patients were randomized to receive tivozanib (0.89 mg) plus nivolumab or tivozanib alone (1.34 mg). The primary endpoint was progression free survival by independent radiology review. The key secondary endpoint was overall survival, and other secondary endpoints included investigator-assessed progression free survival, objective response rate, duration of response, and safety/tolerability. The trial design for TiNivo-2 was as follows:
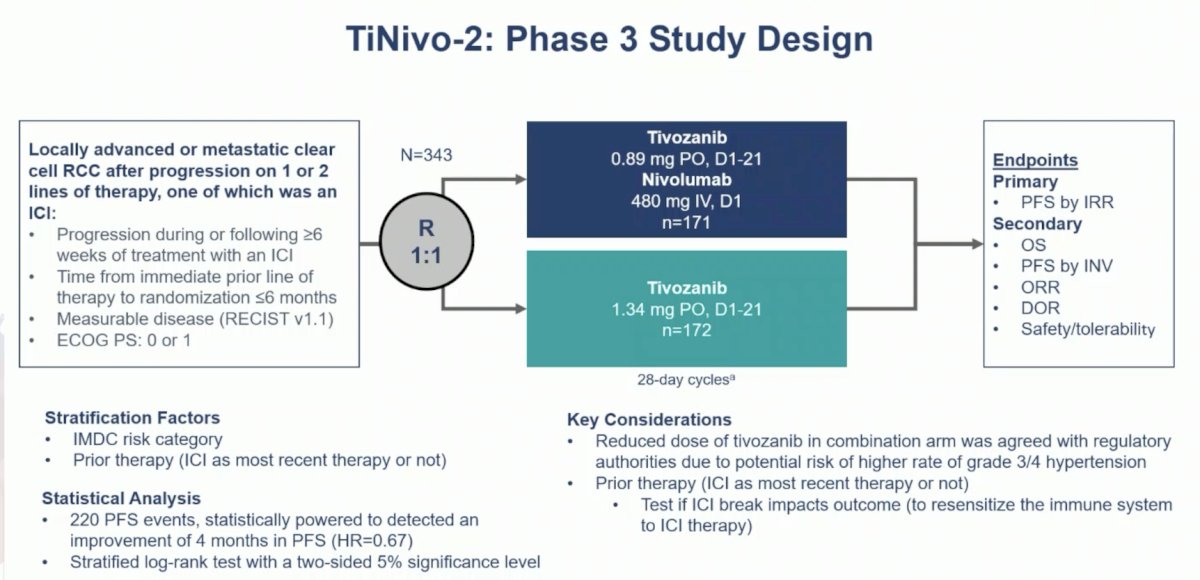
A sample size of 343 patients with 220 events provided ≥80% power to detect a 50% improvement in progression free survival, 12 months versus 8 months, assessed by independent radiology review.
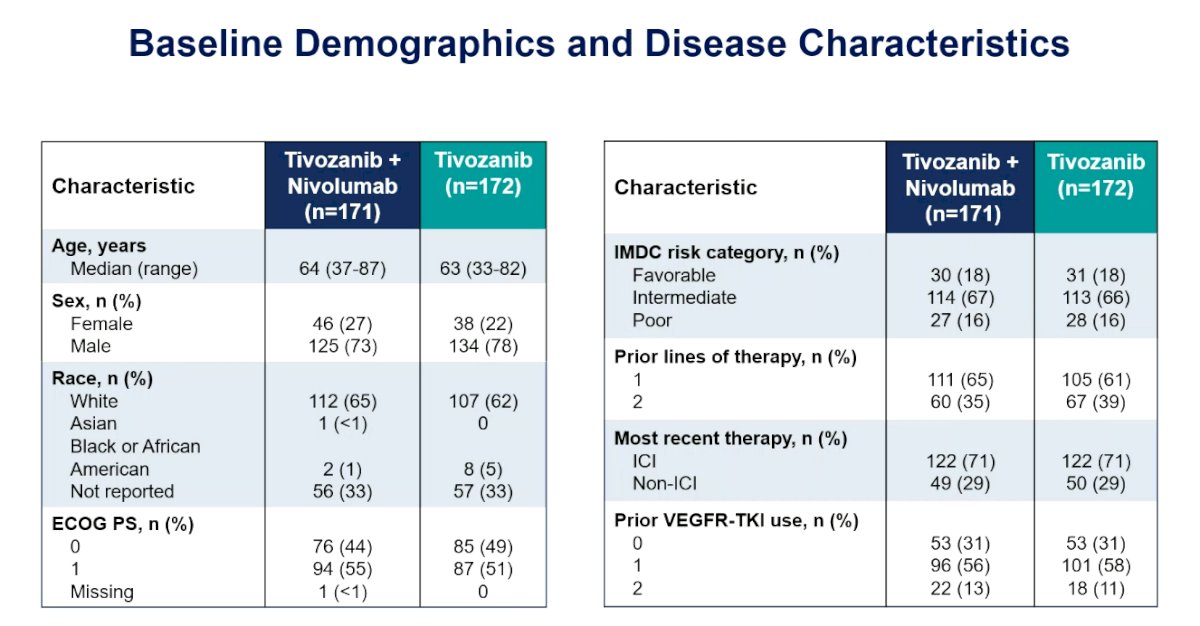
There were 343 patients randomized to tivozanib + nivolumab (n = 171) or tivozanib (n = 172), with well balanced baseline characteristics:
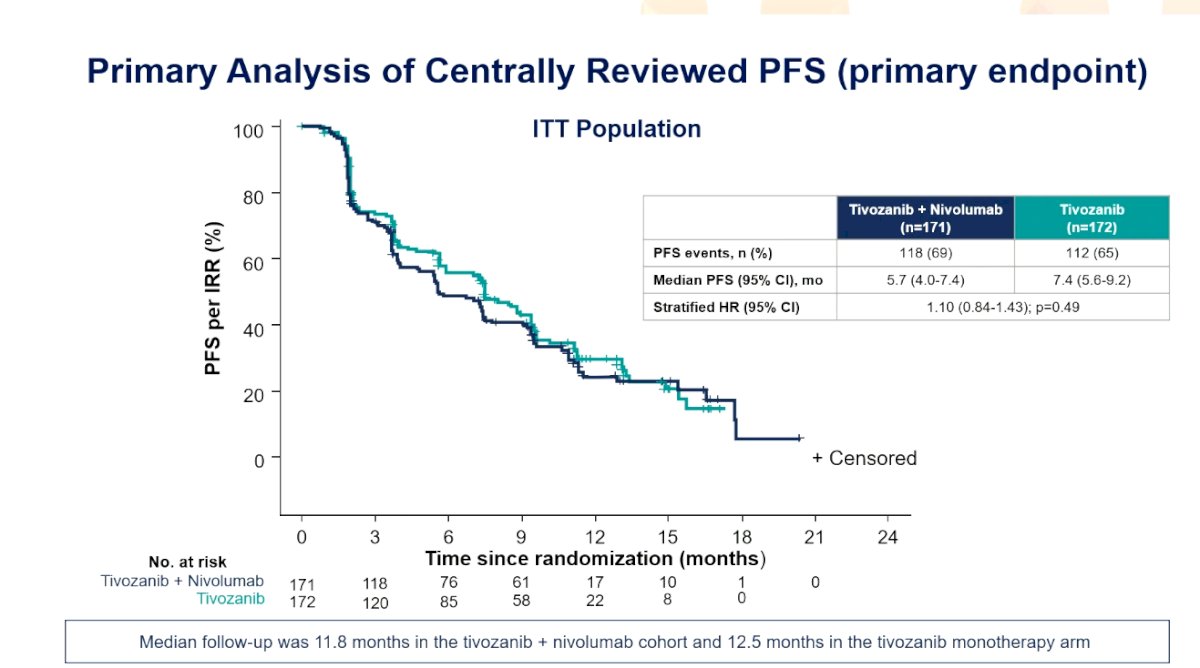
With a median independent radiology review-assessed progression free survival of 5.7 months for tivozanib + nivolumab and 7.4 months for tivozanib (HR 1.10, 95% CI 0.82-1.43), the study did not meet its primary endpoint:
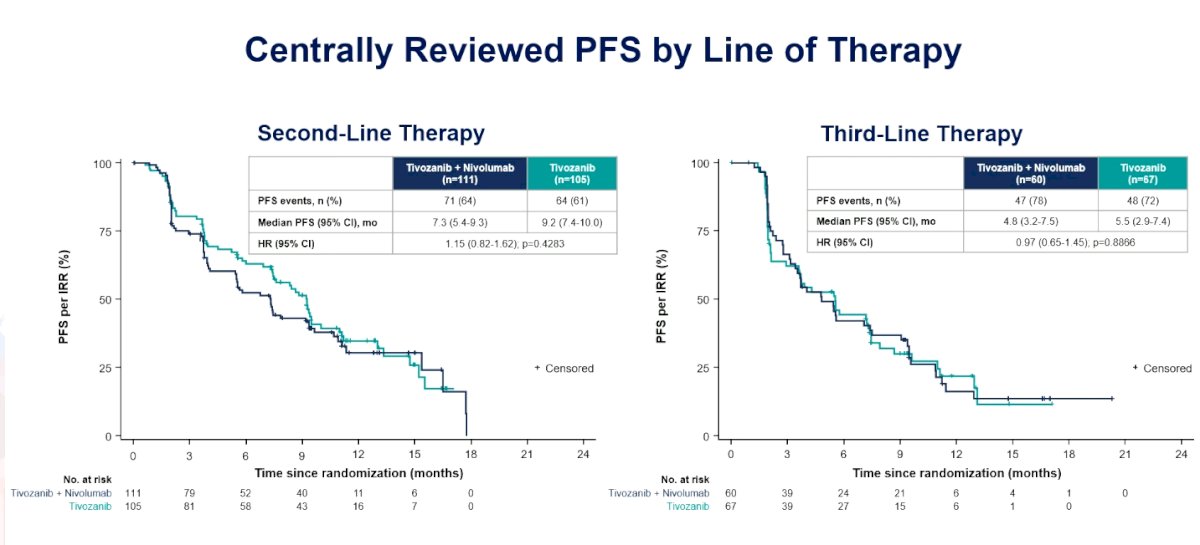
When assessing centrally reviewed progression free survival by line of therapy, there was no benefit in either the second or third line of therapy:
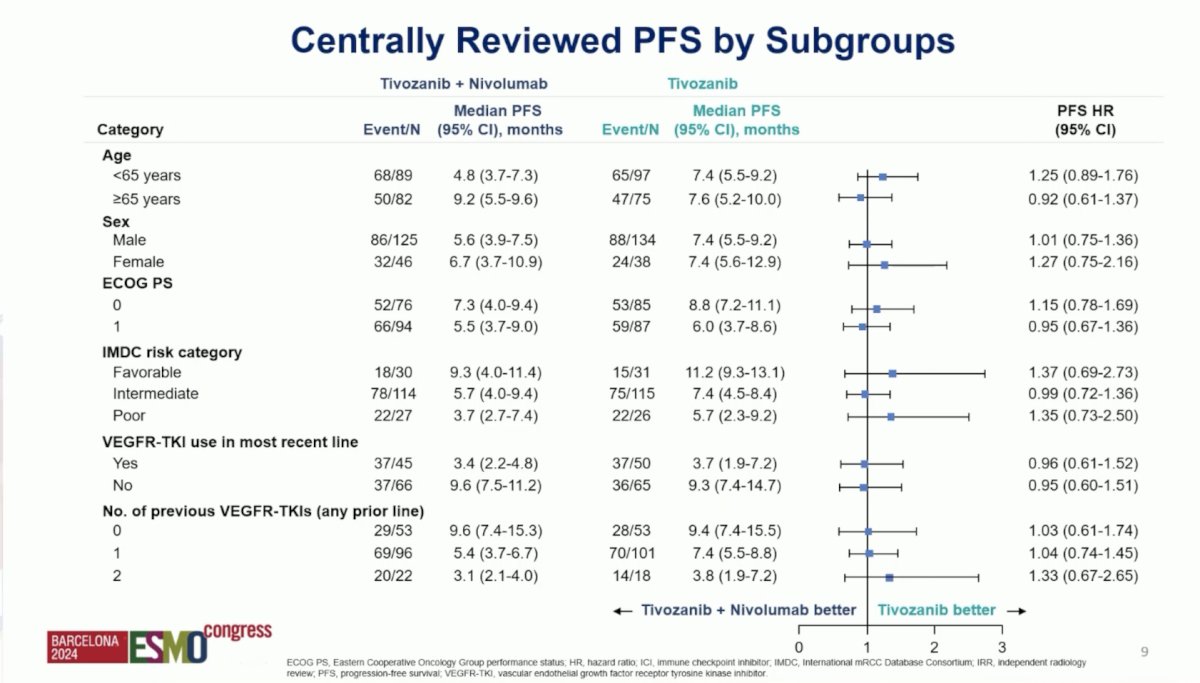
Moreover, when assessing centrally reviewed progression free survival by subgroups, there was no one particular subgroups that appeared to have benefited from tivozanib + nivolumab:
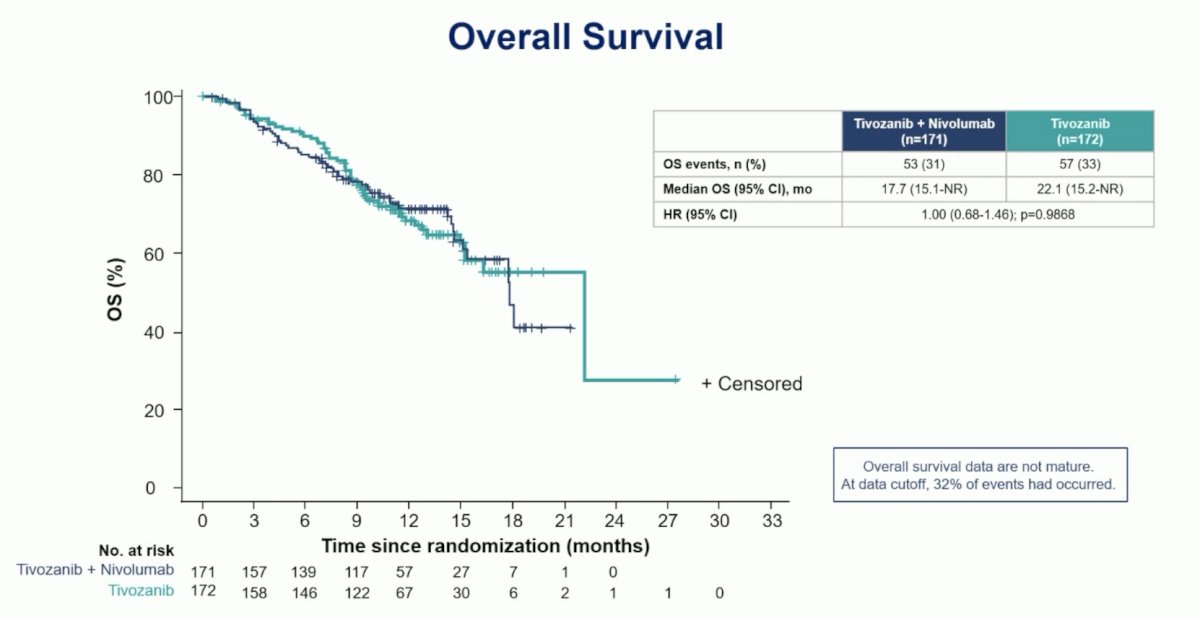
The median overall survival for tivozanib + nivolumab was 17.7 months (95% CI 15.1 – not reached) and for tivozanib monotherapy was 22.1 months (95% CI 15.2 – not reached; HR 1.00, 95% CI 0.68 – 1.46):
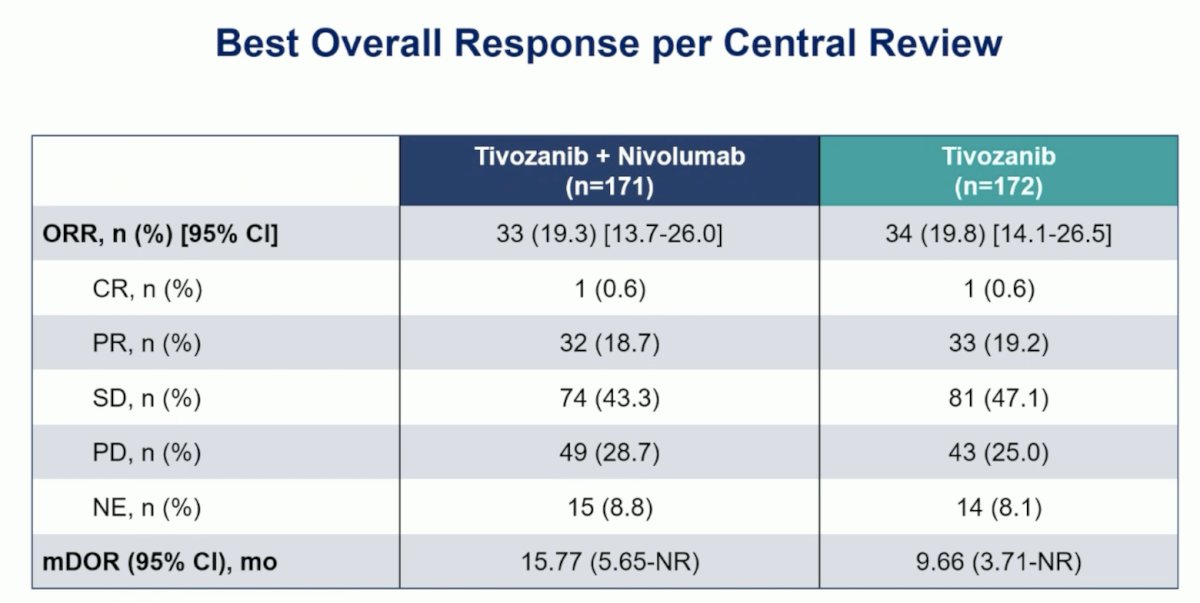
The objective response rate for tivozanib + nivolumab was 19.3% (95% CI 13.7 – 26.0) and for tivozanib was 19.8% (95% CI 14.1 – 26.5), with a median duration of response of 15.77 months (95% CI 5.65 – not reached) versus 9.66 months (95% CI 3.71 – not reached), respectively:
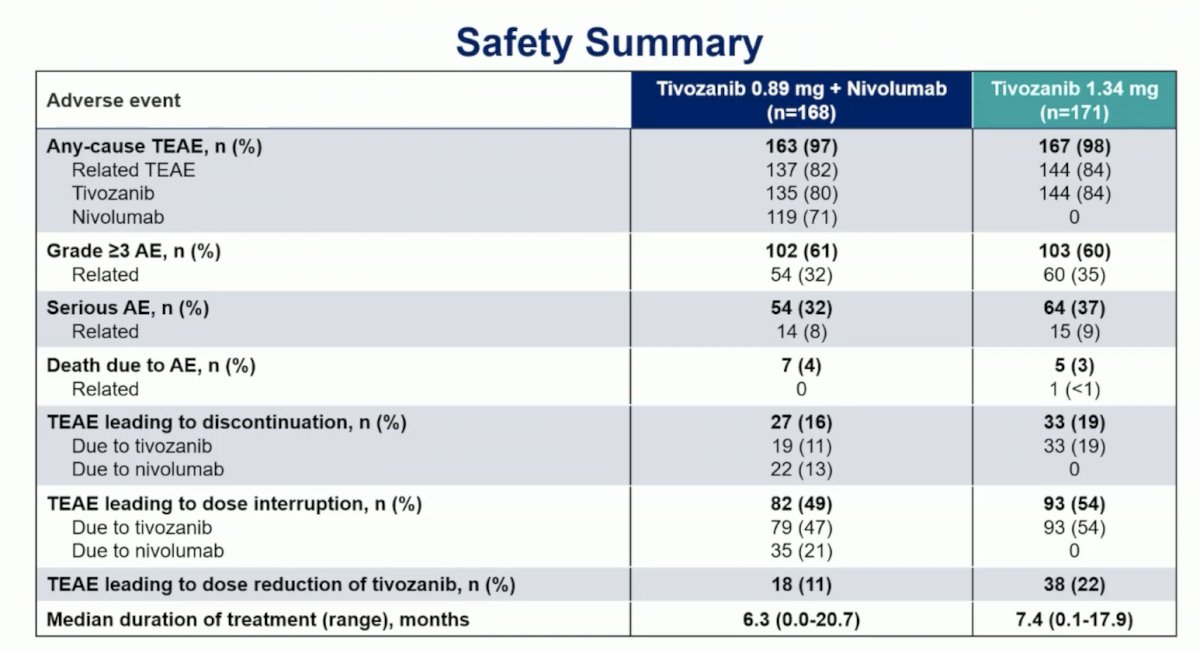
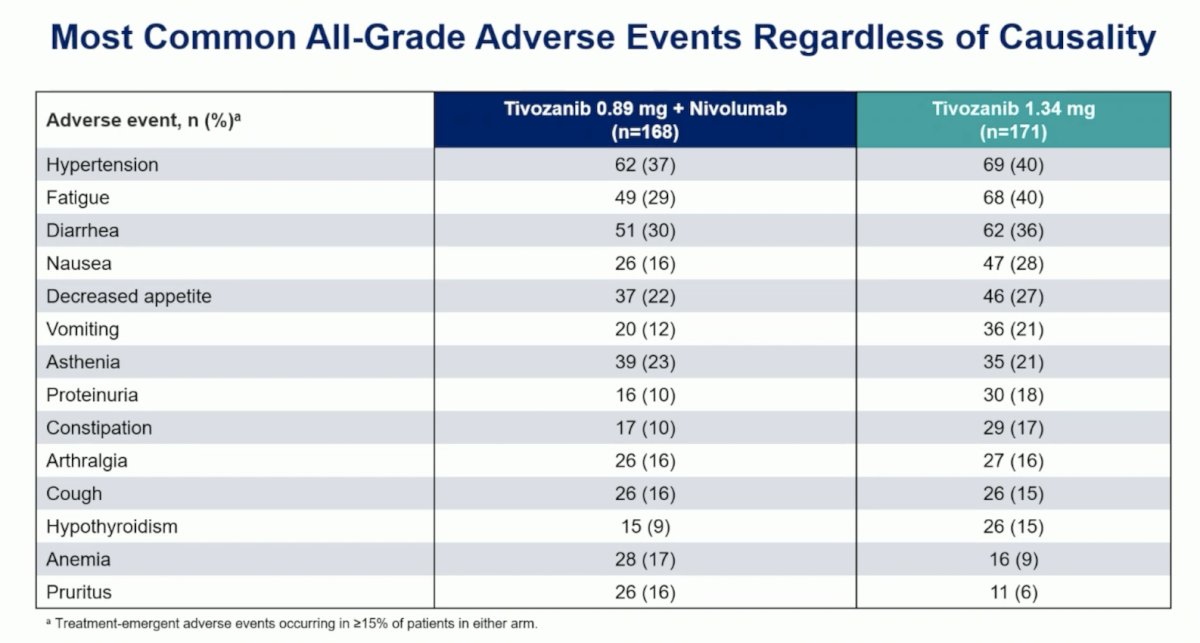
Overall, 205 (60%) patients experienced a grade 3 or higher adverse events, the most common was hypertension (22% both arms; others <5%). Adverse events leading to death occurred in 7 (4.2%) patients with tivozanib + nivolumab and 5 (2.9%) with tivozanib, with 1 death (tivozanib arm) deemed treatment related:
Dr. Choueiri concluded his presentation by discussing results from the phase III TiNivo-2 trial with the following take-home points:
- TiNivo-2 was the first randomized, phase 3 trial to evaluate the efficacy and safety of a PD-1 inhibitor combination following progression on or after prior treatment with PD-1/PD-L1 therapy
- The addition of nivolumab to tivozanib did not result in improved clinical outcomes in patients with metastatic RCC whose disease had progressed on or after prior immune checkpoint inhibitor therapy. Additionally, no subgroup was identified that benefited from the addition of nivolumab
- This trial confirms and expands key conclusions from CONTACT-03 and suggests that immune checkpoint inhibitor rechallenge should be generally discouraged regardless of treatment sequence
- The reduced dose of tivozanib in the combination arm may have impacted the efficacy reflected by the numerically lower median progression free survival
- Meaningful efficacy was observed in the second-line tivozanib monotherapy arm, with a 9.2 month median progression free survival immediately following immune checkpoint inhibitor therapy
- These results support tivozanib monotherapy at 1.34 mg daily as second-line therapy for patients following progression on previous immune checkpoint inhibitor combination therapy
Presented by: Toni K. Choueiri, MD, Dana Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related Content:
TiNivo-2 Trial Results: Tivozanib in Advanced Renal Cell Carcinoma Treatment - Toni Choueiri
Tivozanib plus nivolumab versus tivozanib monotherapy in patients with renal cell carcinoma following an immune checkpoint inhibitor: results of the phase 3 TiNivo-2 Study


