(UroToday.com) The 2024 ESMO annual meeting included a session on kidney cancer, featuring a presentation by Dr. Brian Rini discussing the final analysis of the phase 3 LITESPARK-005 study of belzutifan versus everolimus in participants with previously treated advanced clear cell RCC. Belzutifan is a first-in-class oral HIF-2alpha inhibitor that blocks heterodimerization with HIF-1beta and downstream oncogenic pathways:

Belzutifan significantly improved progression-free survival and objective response rate versus everolimus in patients with advanced clear cell RCC after immune checkpoint and antiangiogenic agents in LITESPARK-005 (NCT04195750).1 Based on these results, belzutifan was approved in the US for advanced RCC following a PD-(L)-1 inhibitor and a VEGFR-TKI. At ESMO 2024, Dr. Rini presented the final results of the LITESPARK-005 trial.
Eligible patients were adults with advanced clear cell RCC and 1–3 prior systemic regimens including ≥1 PD-(L)1 inhibitor and ≥1 VEGFR-TKI. Patients were randomized 1:1 to belzutifan 120 mg or everolimus 10 mg QD until progression or unacceptable adverse events. Progression-free survival per RECIST 1.1 by central review and overall survival were the dual primary endpoints. Objective response rate per RECIST 1.1 by central review was a key secondary endpoint. Duration of response and safety were secondary endpoints. The trial design for LITESPARK-005 is as follows:
No formal statistical testing was performed at final analysis for progression-free survival and objective response rate as these endpoints were positive at first interim analysis.
There were 746 patients allocated to belzutifan (n = 374) or everolimus (n = 372). At final analysis (data cutoff: April 15, 2024), the participant disposition in the two arms was as follows:
The median follow-up was 35.8 months (range 26.9–49.2), with 14.5% of patients having ongoing treatment with belzutifan versus 1.4% with everolimus. Dr. Rini highlighted the baseline characteristics in the intention to treat the population, as well as those that were still receiving ongoing treatment with belzutifan: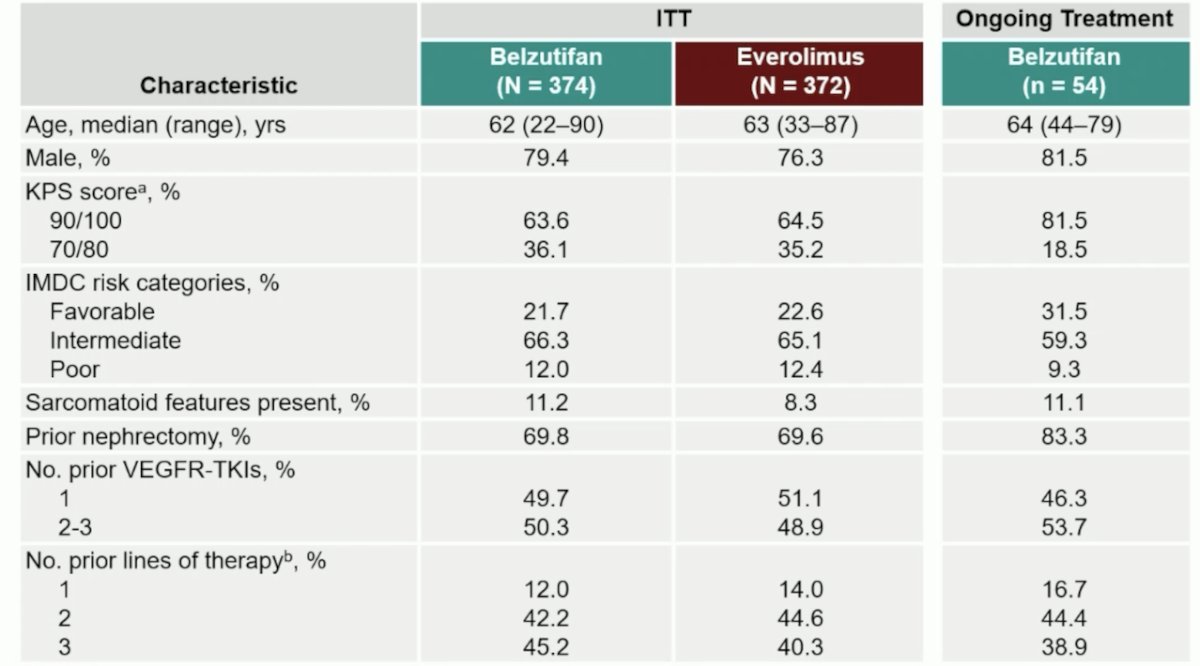
The progression-free survival benefit was maintained with belzutifan vs everolimus (median 5.6 months versus 5.6 months; HR 0.75; 95% CI 0.63–0.88), with an estimated progression-free survival rate at 12 months (33.7% vs 17.6%) and at 24 months (17.5% vs 4.1%) favoring belzutifan:
Progression-free survival across the subgroups generally showed an excellent benefit for belzutifan versus everolimus:
Median overall survival was 21.4 months with belzutifan versus 18.2 months with everolimus (HR 0.92; 95% CI 0.77–1.10; p = 0.18). Estimated overall survival rate was 67.9% vs 65.8% at 12 months and 45.2% vs 41.2% at 24 months:
Objective response rate was consistent with prior reports (22.7% vs 3.5%), and median duration of response was 19.3 months (range: 1.9+–40.1+) versus 13.7 months (range: 3.8–29.5+):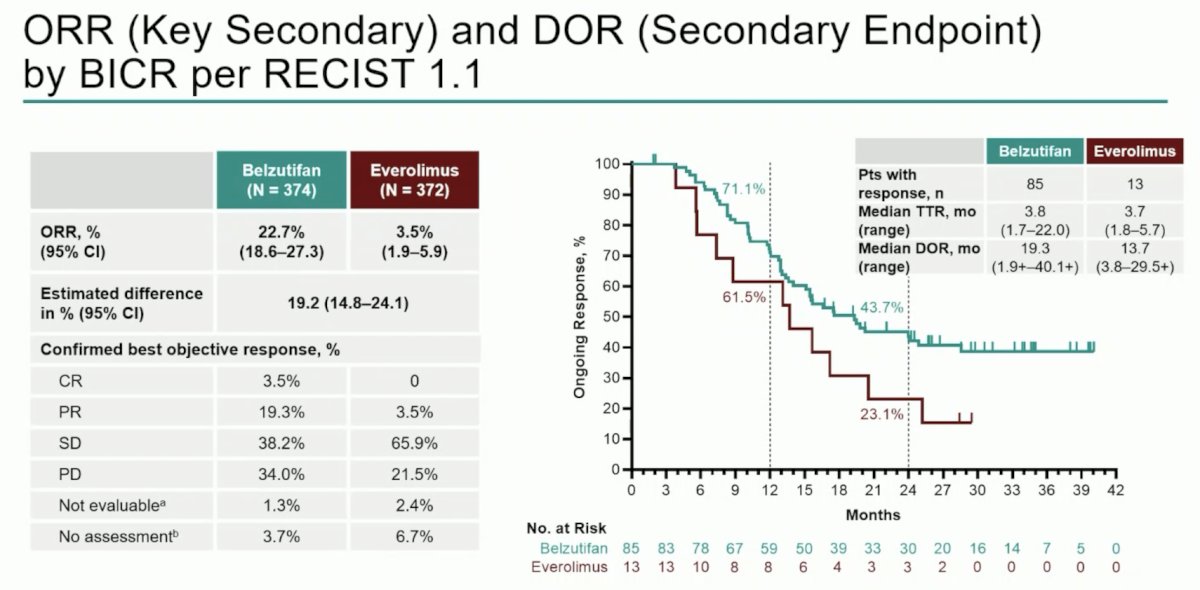
Among patients with no subsequent therapy, there were 173 in the belzutifan arm (61 alive at final analysis) and 121 in the everolimus arm (12 alive at final analysis):
Fewer patients discontinued belzutifan (6.2%) vs everolimus (15.3%) due to any-cause adverse events, and median duration of therapy was longer with belzutifan (7.6 months vs 3.9 months). Rate of grade ≥3 treatment-related adverse events (39.5% vs 40.0%) was similar between arms. The following shows the time to the first onset of common any-grade adverse events attributed to belzutifan (adverse drug reactions):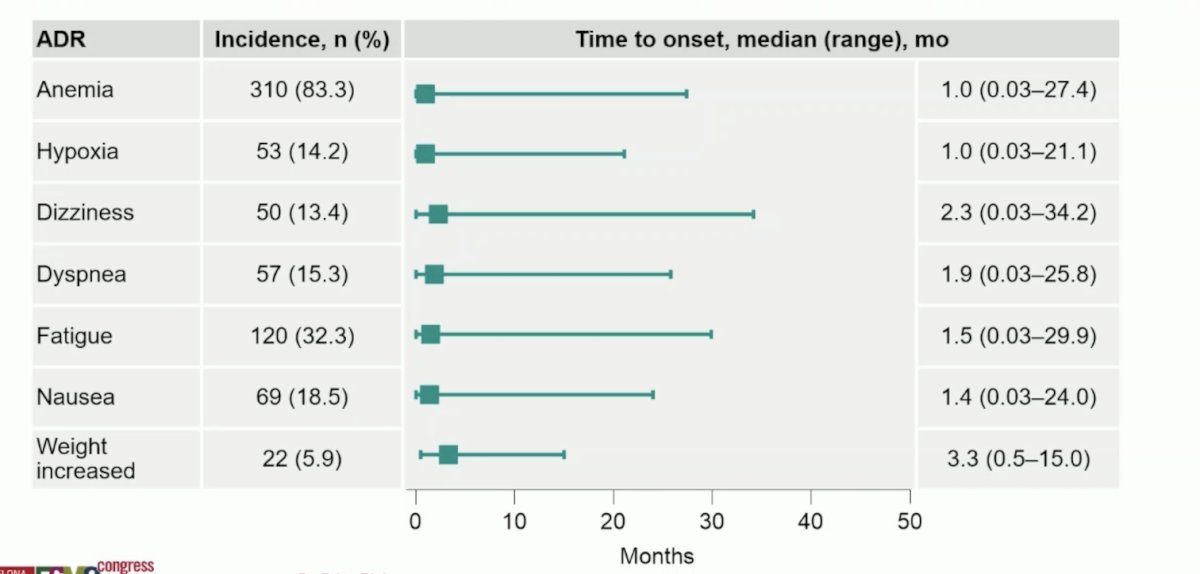
Additionally, the following shows the duration of common any-grade adverse events attributed to belzutifan:
Dr. Rini concluded his presentation discussing the final analysis of the phase 3 LITESPARK-005 trial with the following take-home points:
- At final analysis of the phase 3 LITESPARK-005 study, belzutifan continued to show progression-free survival and objective response rate benefits versus everolimus, including durable response lasting > 2 years
- With >2 years minimum follow-up, more participants remained on treatment with belzutifan versus everolimus
- Significant improvement in overall survival was not observed
- There were no new safety signals for belzutifan observed. The most common any-grade adverse drug reactions had a median time to onset of < 2 months.
- Final analysis results of LITESPARK-005 support belzutifan as a treatment option in advanced clear cell RCC after PD-(L)1 inhibitor and VEGFR-TKI therapy
Presented by: Brian I. Rini, MD, FASCO, Chief of Clinical Trials, Vanderbilt University, Nashville, TN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:


