(UroToday.com) The 2024 ESMO annual meeting included a session on kidney cancer, featuring a discussant presentation by Dr. Manuela Schmidinger discussing three abstracts including “Tivozanib–Nivolumab vs Tivozanib Monotherapy in Patients with RCC Following 1 or 2 Prior Therapies including an Immune Checkpoint Inhibitor – Results of the Phase III TiNivo-2 Study” by Dr. Toni Choueiri, “Final analysis of the phase 3 LITESPARK-005 study of belzutifan versus everolimus in participants with previously treated advanced clear cell RCC” by Dr. Brian Rini, and “Prospective randomized phase II trial of Ipilimumab + Nivolumab versus standard of care in non-clear cell RCC: Results of the SUNNIFORECAST trial” by Dr. Lothar Bergmann.
Dr. Schmidinger notes that the guidelines suggest that after a patient has an IO therapy failure, a VEGFR systemic therapy that has not been given previously should be used as next line of therapy. Based on CONTACT-03,1 the agent of choice was cabozantinib, but we must continue to aspire for durable responses, which seem achievable only with IO-based therapies. TiNivo-2 is a phase III trial and a second attempt to demonstrate the benefit of IO after IO failure:
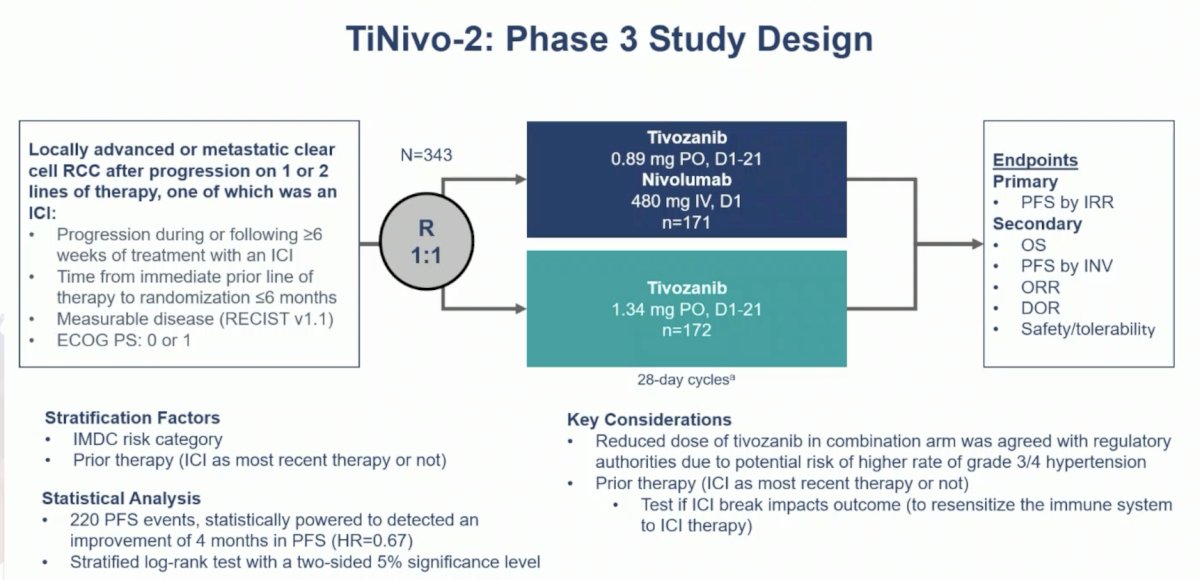
The key findings of TiNivo-2 were that tivozanib + nivolumab is not superior to tivozanib monotherapy in terms of progression free survival, objective response rate, complete response rate, or overall survival. In fact, there was no subgroup that demonstrated benefit from the combination, however, duration of response was longer in the tivozanib + nivolumab arm. According to Dr. Schmidinger, 3 major questions arise from this data:
- Was the trial design able to provide a valid answer to the question of whether IO should be given after IO?
- Is it time to say goodbye to IO after IO?
- Is tivozanib a new standard of care after IO?
The dose of tivozanib was reduced to 50% in the combination arm, which was agreed upon with regulatory authorities, due to the potential risk of high grade hypertension. However, Dr. Schmidinger notes that it’s unlikely that hypertension would be more of an issue when tivozanib is combined with nivolumab. Hypertension is usually very well managed and a key adverse event linked to efficacy and appropriate drug exposure. Furthermore, the maximum tolerated dose of tivozanib in all combination trials across several malignancies was defined as 1.5 mg daily.
So, can we conclude that tivozanib + nivolumab failed to improve outcomes versus tivozanib alone after prior IO? Or was it rather that the addition of nivolumab was unable to overcome the disadvantage of an under-dosed tivozanib regimen? At ASCO 2023, Dr. David Braun suggested in the discussion of CONTACT-03 that “due to long-term receptor occupancy, the trial might essentially represent a comparison of cabozantinib + atezolizumab versus cabozantinib + persistent effect of preceding immune checkpoint inhibitor.” As such, Dr. Schmidinger suggests that this may also be a solid argument for the tivozanib + nivolumab combination in TiNivo-2, especially given that 71% of patients had IO as the immediately preceding therapy.
Is tivozanib a new standard of care after IO-failure? Indeed, efficacy is present, but according to Dr. Schmidinger, it looks inferior to cabozantinib or lenvatinib + pembrolizumab:
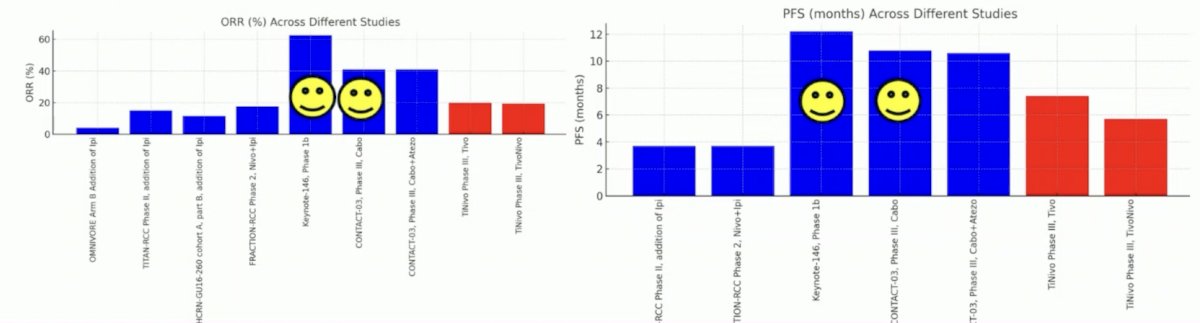
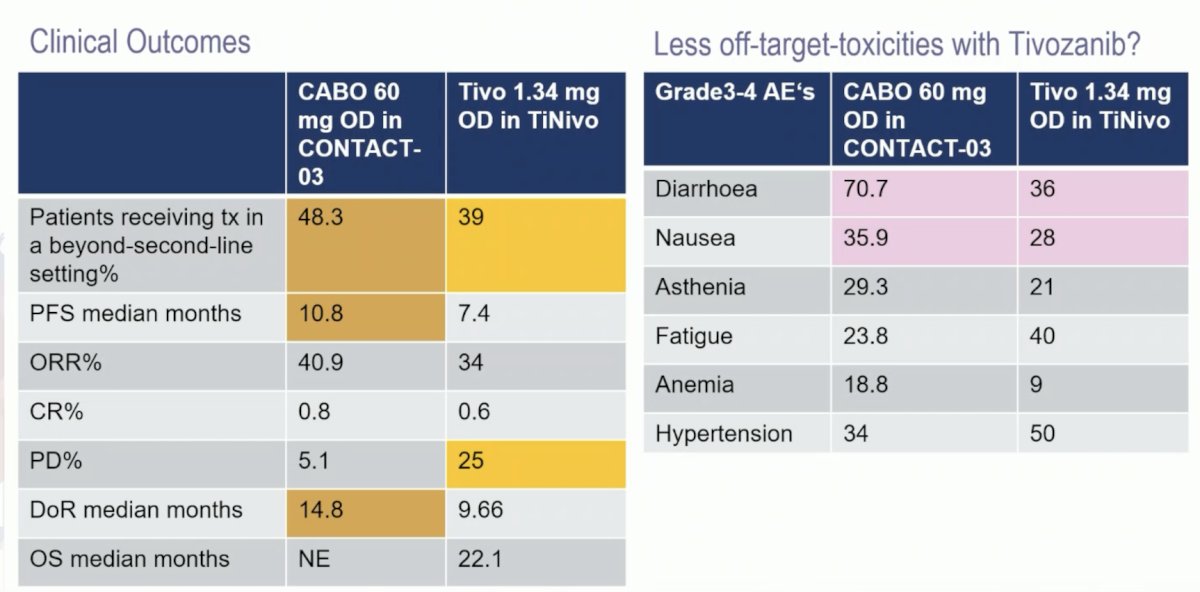
Cabozantinib is a broader TKI (VEGF-MET-AXL) and more potent in inhibiting VEGFR-2 (ICI50: 0.035 versus 0.21 nmol), objective response rates are higher, and progression free survival is longer, which may be promising. Lenvatinib + pembrolizumab remains highly attractive, but phase III data is still lacking. The following is a descriptive comparison between cabozantinib and tivozanib after IO:
Dr. Schmidinger concluded her discussion of TiNivo-2 with the following thoughts:
- The dose of tivozanib in this trial hindered the ability to answer the scientific question
- The maximum tolerated dose for tivozanib has been well established in several combination trials and is either 1.5 or 1.34 mg
- When we refer to the efficacy of IO-TKI combinations, we typically assume a full dose TKI regimen
- Dr. Braun’s point on PD-1 receptor occupancy remains an issue when investigating IO after IO
- Single agent, full dose tivozanib is efficacious after IO, it is another standard of care option besides cabozantinib, with less off target toxicities reported
- Other options like belzutifan and combinations are entering the field and the second line space will be as crowed as the first line space
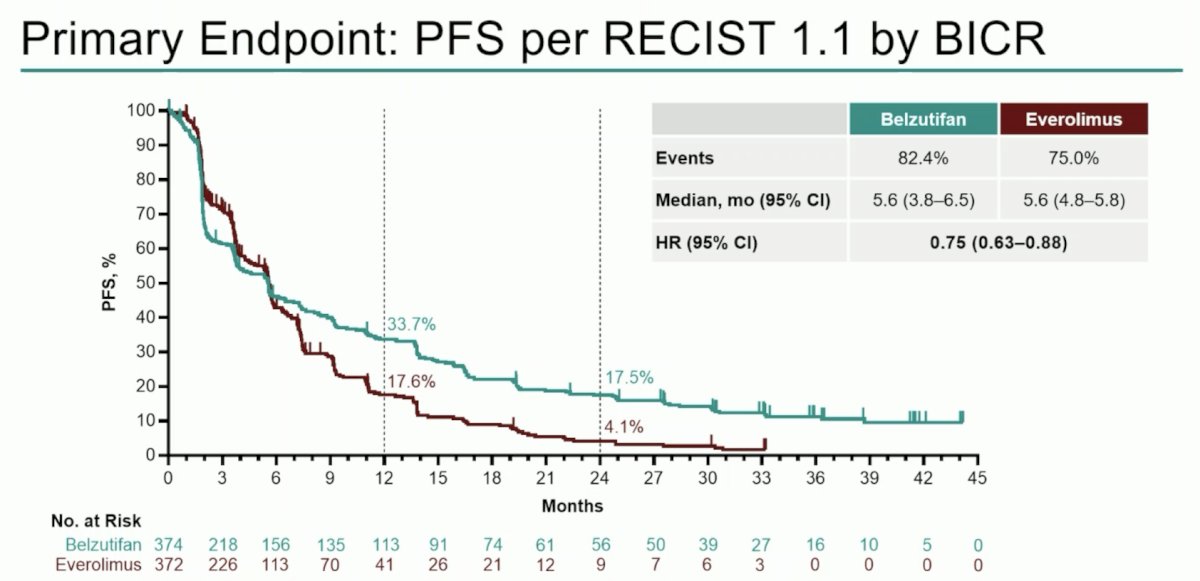
Next, Dr. Schmidinger discussed the final analysis of the LITESPARKK-005 study, which essentially asks the question: Can we advance the post IO setting without TKIs and IO? LITESPARK-005 is a positive trial, with a progression-free survival benefit maintained with belzutifan versus everolimus (median 5.6 months versus 5.6 months; HR 0.75; 95% CI 0.63–0.88), with an estimated progression-free survival rate at 12 months (33.7% vs 17.6%) and at 24 months (17.5% vs 4.1%) favoring belzutifan:
Median overall survival was 21.4 months with belzutifan versus 18.2 months with everolimus (HR 0.92; 95% CI 0.77–1.10; p = 0.18). Estimated overall survival rate was 67.9% vs 65.8% at 12 months and 45.2% vs 41.2% at 24 months:
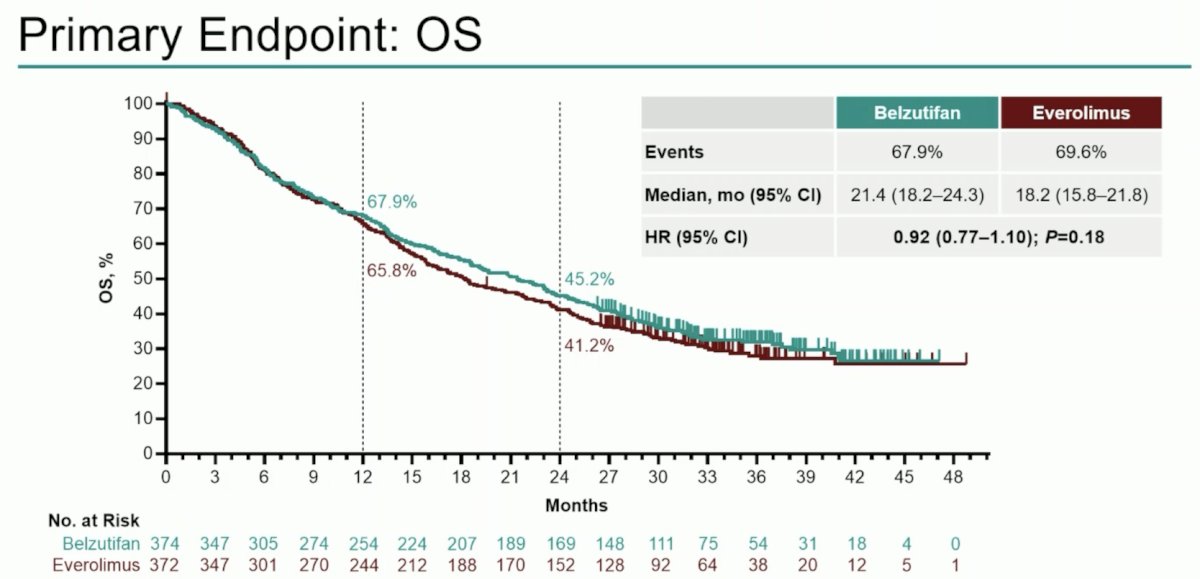
Objective response rate was consistent with prior reports (22.7% vs 3.5%), and median duration of response was 19.3 months (range: 1.9+–40.1+) versus 13.7 months (range: 3.8–29.5+):
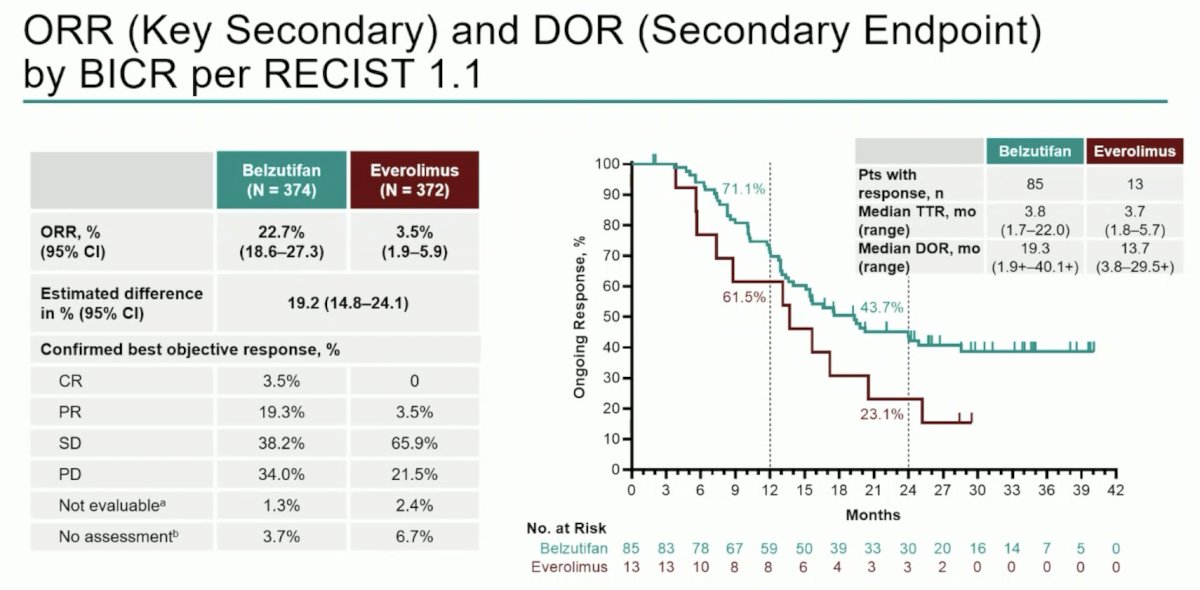
Dr. Schmidinger states that belzutifan compared to other therapies in the post-IO space has solid efficacy, but is not a new benchmark. The following compares objective response rates, progression free survival, and overall survival among post-IO options:
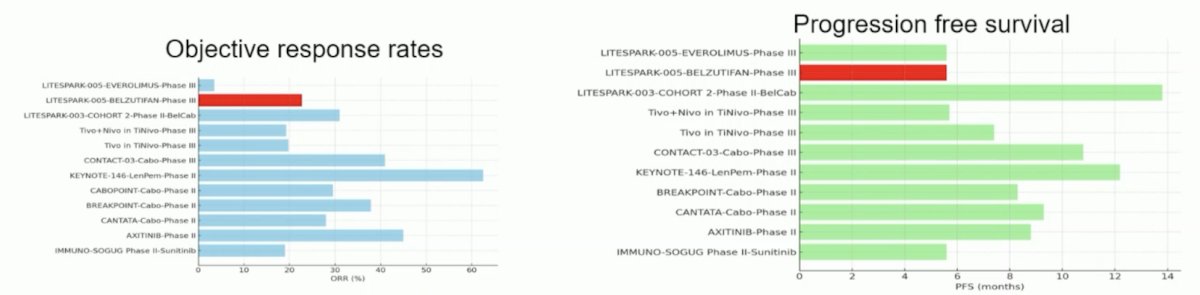
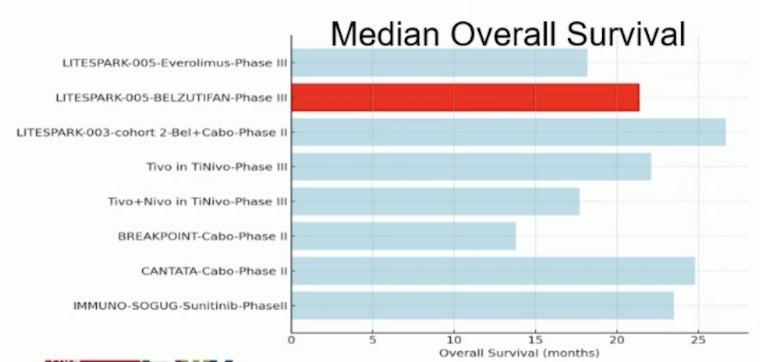
Dr. Schmidinger notes that LITESPARK-005 is the only trial, in which almost half (45.2%) of the patients were in a 4th-line setting. Strategies with higher objective response rates or longer progression free survival had either less pretreated patients and/or were combinations.
Is overall survival improvement possible in pretreated patients? Yes, METEOR2 and CheckMate-0253 have shown overall survival benefits. While over 70% of patients in these trials were treated in a second line setting, only 12% in LITESPARK-005 were second line therapy patients. While a significant overall survival benefit may have been an idealized expectation, the challenge for this trial was not the comparator, but rather the stage of disease. The later the stage, the more intratumor heterogeneity there is, as well as accumulation of genetic alterations and adaptive resistance. Thus, the more prior therapies a patient receives, the more pressure there is to select resistant clones.
Dr. Schmidinger concluded her discussion of LITESPARK-005 with the following thoughts:
- Although there is no benefit for overall survival, belzutifan still makes a substantial difference
- There are benefits in objective response rate, duration of response, progression free survival, duration of therapy, and less need for subsequent therapies
- There is a significant delay of deterioration (19 versus 10 months), as well as untold psychological benefits
- Should it be used earlier in the disease course? Probably
- Belzutifan is the only targeted agent that can be combined at a full dose with a TKI
- Belzutifan combinations may drive the next major RCC breakthrough
Finally, Dr. Schmidinger discussed the role of IO-doublet therapies in the first-line management of non clear cell RCC. The SUNNIFORECAST trial is a classic basket trial population, with papillary and chromophobe histology the most common subtypes; sarcomatoid histology was ~5% in each arm. The SUNNIFORECAST trial design is as follows:
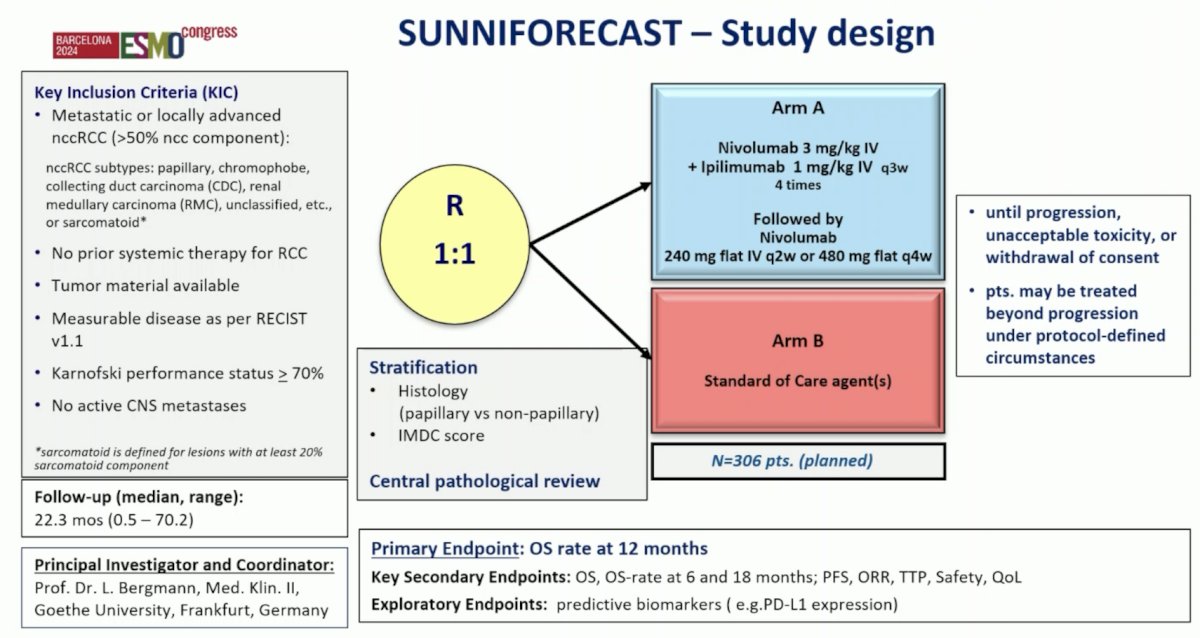
Over a median follow-up of 24.3 months, Dr. Schmidinger’s key findings from this trial of nivolumab + ipilimumab versus standard of care in non clear cell RCC are as follows:
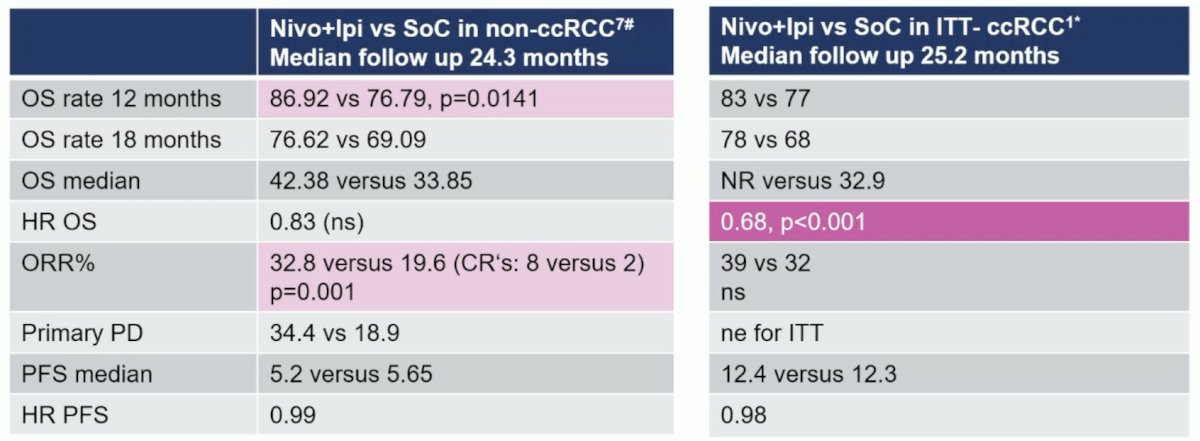
- 12 month overall survival rate: 86.92% versus 76.79%, p = 0.0141
- 18 month overall survival rate: 76.62 versus 69.09
- Median overall survival: 42.38 versus 33.85 months, HR 0.83, p > 0.05
- Objective response rate: 32.8 % versus 19.8%, p = 0.001
- Median progression free survival: 5.2 versus 5.65 months
She notes that this is interesting data, but not the same as seen in clear cell RCC:
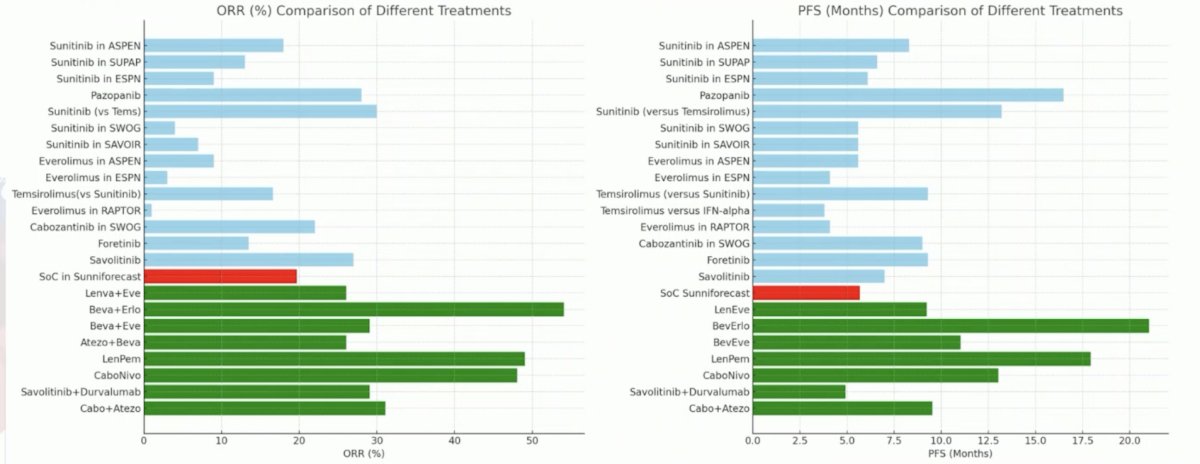
Dr. Schmidinger emphasized that the standard of care arm may have underperformed in SUNNIFORECAST, but it is difficult to compare secondary to trial heterogeneity:
It is important to remember that nivolumab + ipilimumab is already a guideline recommended therapy (IIIA) for patients with non clear cell + non-papillary + predominantly sarcomatoid RCC.
There are still some uncertainties for PD-L1 in the SUNNIFORECAST trial given that standard of care treatment was particularly disadvantageous in PD-L1 positive patients, where the median overall survival was significantly improved with nivolumab + ipilimumab (p = 0.03). Moreover, we do not know how patients were treated in the standard of care arm, but heterogeneity is likely. Among 299 patients receiving study medication, 86.7% had a single agent TKI, 11.8% an IO + TKI. We can make assumptions about the standard of care choice based on subsequent treatments, which indicate that 40% did not receive cabozantinib as a standard of care in the first line, although the majority of patients had papillary RCC:
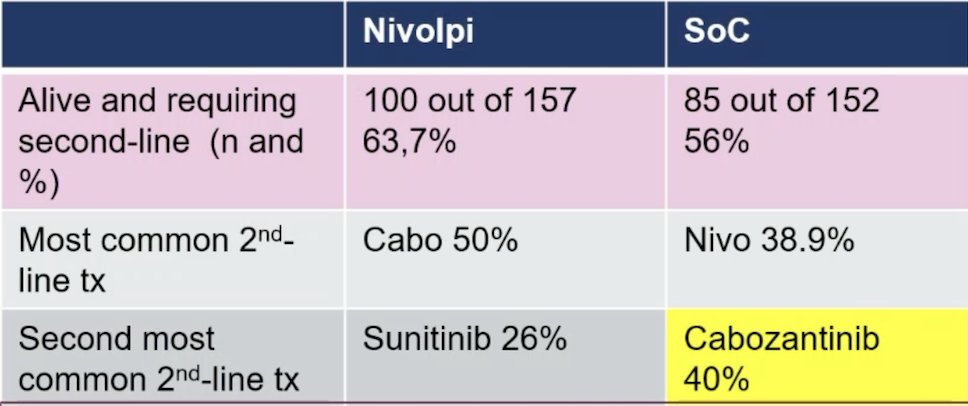
Dr. Schmidinger concluded her discussion of SUNNIFORECAST with the following thoughts:
- These data are interesting and encouraging, but in contrast to clear cell RCC, improved overall survival rate is not maintained with longer follow-up
- This trial suffers from double-heterogeneity: (i) mix of variant histologies, (ii) mix of treatments in the comparator arm
- In at least 40% of patients, cabozantinib was not the standard of care, although most patients had papillary RCC
- There is missing objective response rate data from 17% of the treated population
- There is imbalance in PD-L1 expression (higher in the standard of care arm), which may have caused underperformance of the standard of care arm
- The role of CTLA-4 inhibition in non clear cell RCC beyond sarcomatoid remains a bit vague, but IO + TKI combinations appear more encouraging
Presented by: Manuela Schmidinger, MD, University of Vienna, Vienna, Austria
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Pal SK, Albiges L, Tomczak P, et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicenter, randomized, open-labl, phase 3 trial. Lancet 2023 Jul 15;402(10397):185-195.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1814-1823.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1803-1813.


