(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Sarmad Sadeghi presented the final results of a phase II double-blind, randomized trial of axitinib +/- PF 04518600, an OX40 antibody for metastatic renal cell carcinoma (RCC) patients with disease progression following anti-PD-1/L-1 therapy.
Dr. Sadeghi noted that both primary and acquired resistance to immunotherapy result in poor patient outcomes. Co-stimulatory OX40 (CD-134) activates exhausted T-cells, increases dendritic cell proliferation and effector functions, and, thus, the survival of T cells. PFOX is an OX40 agonist. The study investigators hypothesized that the combination of axitinib + PFOX would improve outcomes following prior anti-PD-(L)1 therapy, compared to axitinib + placebo,
This was a double-blind, placebo-controlled, phase II randomized trial of patients with advanced or metastatic predominantly clear cell RCC with disease progression during or following anti-PD-(L)-1 therapy. Eligible patients underwent 1:1 randomization, stratified by IMDC risk group, to either:
- Arm 1: Axitinib 5 mg orally twice daily + PFOX 0.3 mg/kg intravenously on day 1 of a two-week cycle
- Arm 2: Axitinib + placebo
The primary endpoint was progression-free survival. Secondary endpoints included:
- Overall survival
- Objective response rate
- Duration of response
- Safety and tolerability
A total of 60 patients were recruited between February 2018 and August 2021. The median patient age was 62 years, and 97% had ECOG performance status 0–1. 85% of patients had MSKCC intermediate- to poor-risk disease. 58% of patients had received 1–2 prior lines of therapy, and 42% had received ≥3. 87% of patients had undergone a prior nephrectomy. The most common sites of metastatic disease were the lungs (63%), lymph nodes (47%), and bones (35%).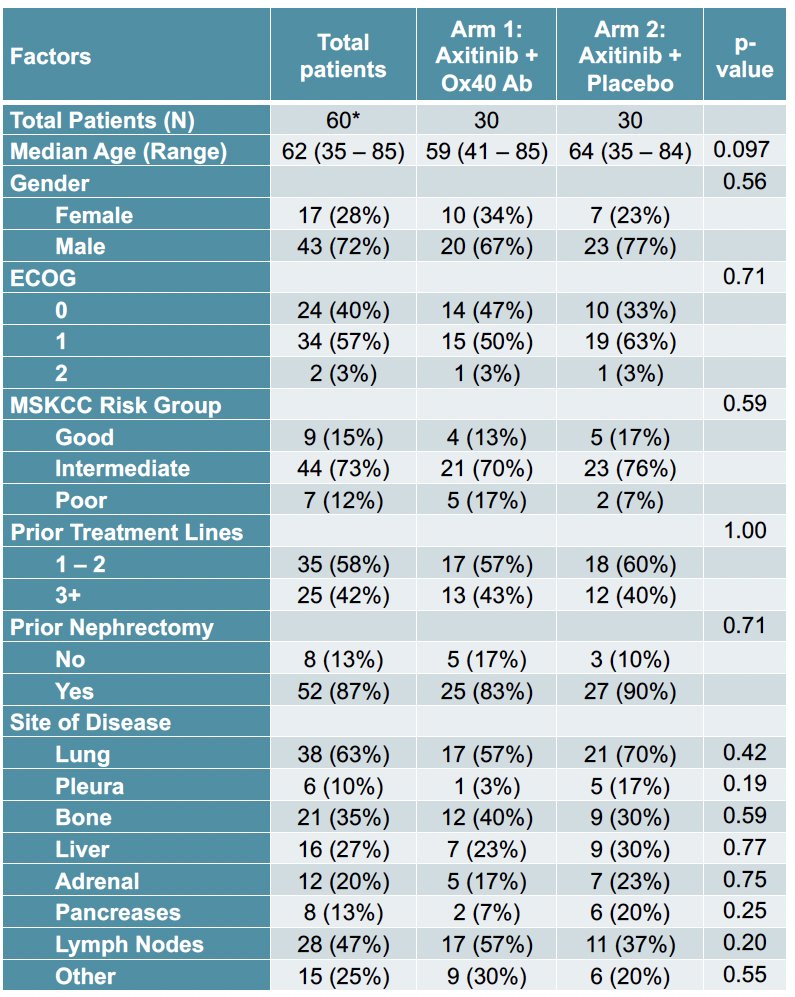
The median follow-up was 38 months. A median of 18 cycles were administered (experimental: 23; control: 17.5 cycles). Dose modifications were required in 43% and 37% of patients in the experimental and control arms, respectively (p=0.79). Grade 3–4 toxicity was observed in 80% and 57% of patients in the experimental and control arms, respectively.
The median progression-free survival was 9.5 (95% CI: 5.9–14.5) versus 8.5 months (95% CI: 5.9–11; HR: 1.02, 95% CI: 0.59–1.78, p=0.92) in the experimental and control arms, respectively.
There were no differences in the overall survival (median, 27.8 versus 35.7 months; p=0.56) and objective response rates (30% versus 37%, p=0.78).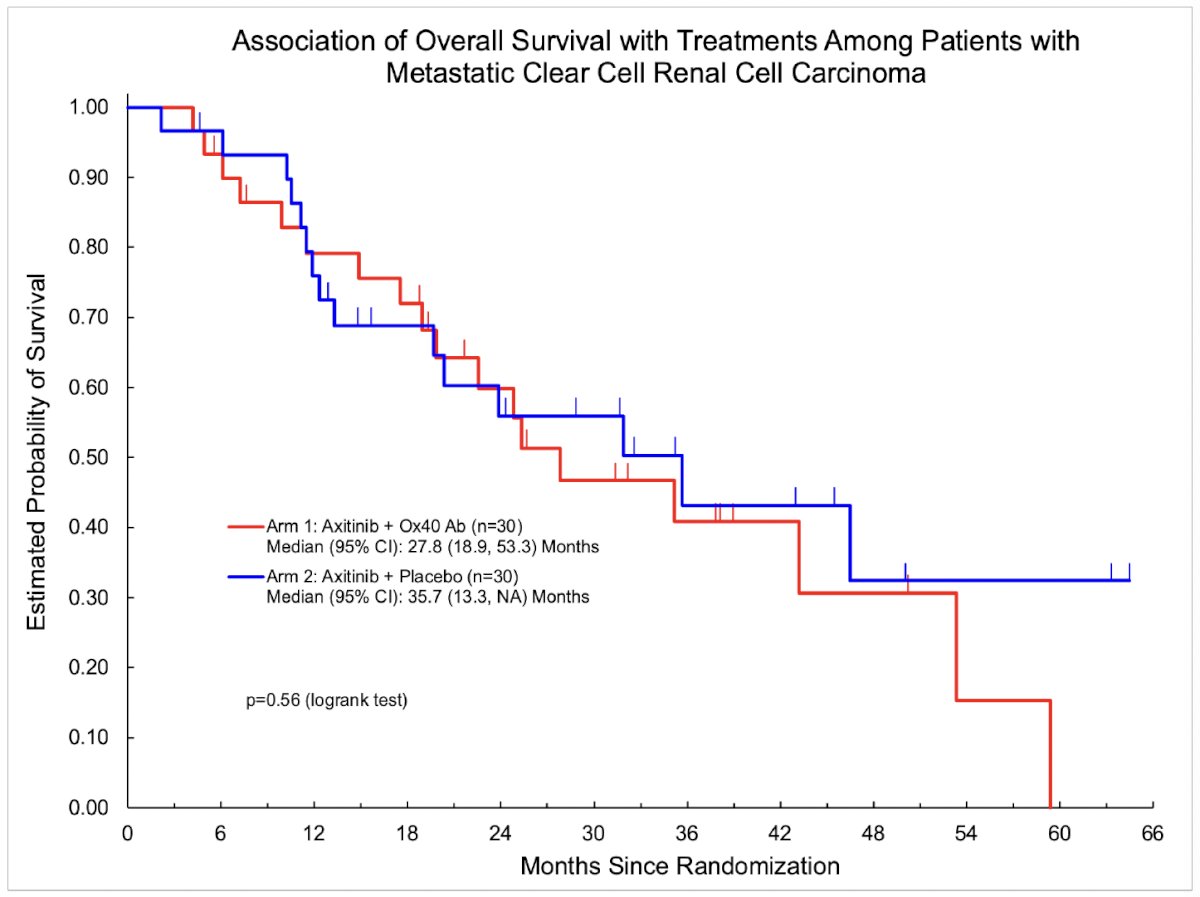
Based on these results, Dr. Sadeghi concluded as follows:
- In immunotherapy-treated metastatic RCC patients, axitinib + PFOX did not improve outcomes, compared to axitinib alone.
- Successful targeting of OX40 requires further investigation in preclinical and clinical models.
Presented by: Sarmad Sadeghi, MD, PhD, Associate Professor, Department of Medicine, University of Southern California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


