(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to presentation of Poster 1691. Dr. Laurence Albiges presented the updated efficacy and safety results from CheckMate 67T evaluating subcutaneous nivolumab (NIVO SC) vs intravenous nivolumab (NIVO IV) in patients with previously treated advanced or metastatic clear cell renal cell carcinoma (ccRCC).
There is an unmet need in oncology for alternative administration options to systemic therapy that improve patients' treatment experiences and optimize healthcare efficiency. The CheckMate 67T study hypothesizes that subcutaneous (SC) delivery may address these challenges and it is typically preferred by patients over intravenous (IV) delivery.1 Nivolumab, when given SC, is co-formulated with recombinant human hyaluronidase PH20 (rHuPH20), which degrades hyaluronan in the extracellular matrix around the SC injection area, enabling the administration of large volumes of the drug.2
Preclinical studies have shown that rHuPH20 transiently increases the dispersion of injected molecules; 24 hours after rHuPH20 administration, dispersion of injected molecules returned to baseline.3
CheckMate 67T (NCT04810078) is a multicenter, randomized, open-label, phase 3 study that evaluated the pharmacokinetics and objective response rate (ORR) non-inferiority of NIVO SC versus IV in patients with locally advanced or metastatic ccRCC treated in the 2nd or 3rd line settings.
This non-inferiority randomized trial included patients with advanced or metastatic clear cell RCC that progressed during or after receiving 1-2 prior systemic regimens and had not received prior immune-oncology therapy. Patients underwent 1:1 randomization to either NIVO SC 1,200 mg + rHuPH20 every 4 weeks (n=248) or NIVO 3mg/kg every 2 weeks (n=247). Treatment was continued until disease progression, unacceptable toxicity, consent withdrawal, completion of two years of treatment, or death. The design of the study is illustrated in the figure below.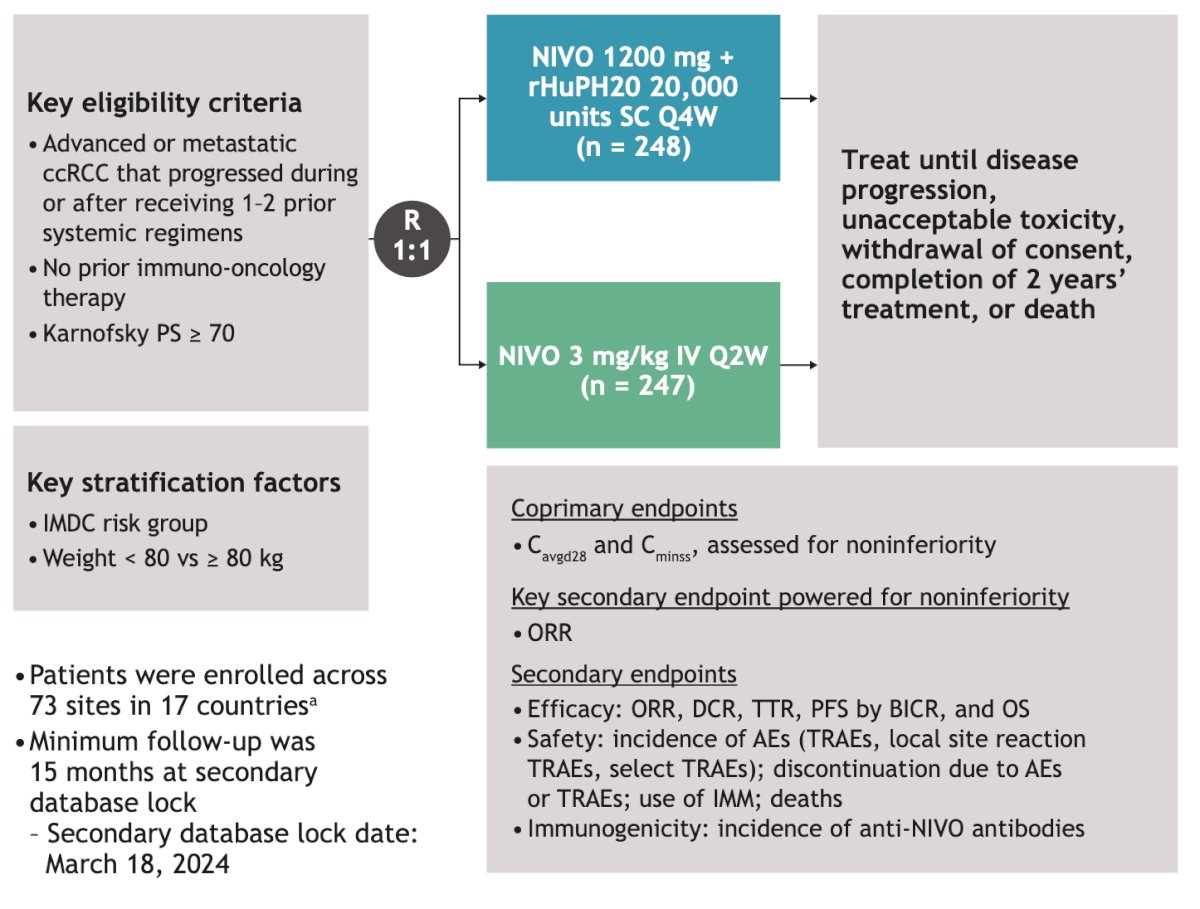
Data from CheckMate 67T, previously presented at ASCO GU 2024, showed that Nivolumab SC was associated with noninferiority in exposures (Cavgd28 and Cminss) and efficacy (overall response rate [ORR] by blinded independent central review [BICR]) compared to Nivolumab IV. Additionally, progression-free survival (PFS) was similar between the SC and IV arms, and safety was consistent between the two delivery methods. Dr. Albiges presented the updated efficacy, safety, and immunogenicity data after a minimum follow-up of 15 months.
Dr. Albiges reported that responses by BICR were comparable between Nivolumab SC and Nivolumab IV: the ORR was 26.6% vs. 20.6%, respectively. Complete responses were achieved by 2% in the SC group and 2.8% in the IV group. The disease control rate (DCR) was 62.5% vs. 64.8% in the SC and IV groups, respectively. Median PFS and Overall survival at 6 and 12 months were similar between both groups. The details are illustrated in the table below.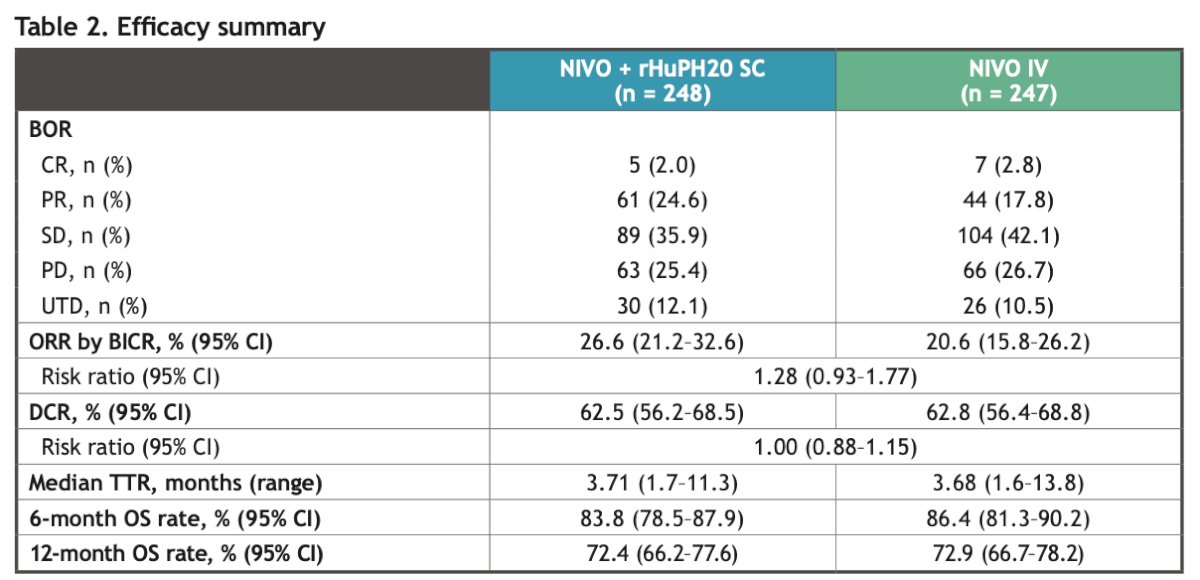
The median PFS was 6.34 months in the Nivolumab SC group vs. 5.65 months in the Nivolumab IV group. However, this difference was not significant.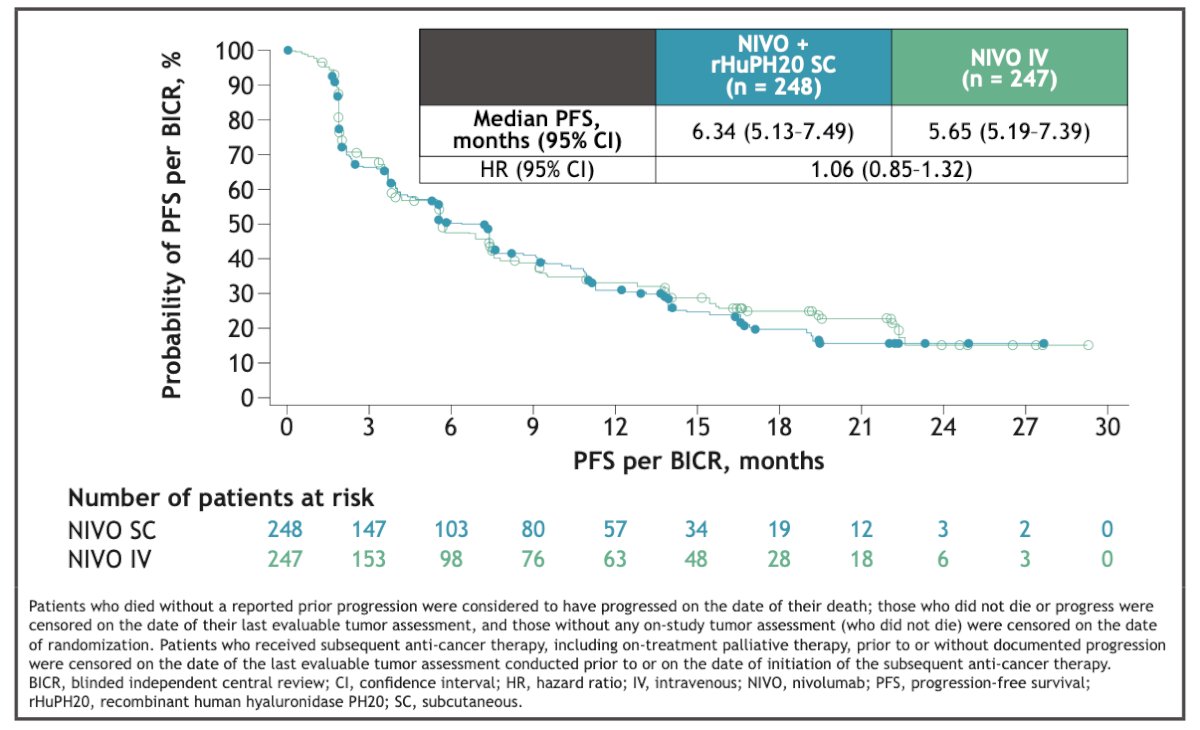
There were no new safety signals observed after 15 months of follow-up. The safety profile of Nivolumab SC was consistent with that of Nivolumab IV. There were three deaths due to study drug toxicity in the Nivolumab SC arm (myocarditis, myasthenia, colitis) and two deaths in the Nivolumab IV arm (immune-mediated pneumonitis/pneumocystis jirovecii bronchopneumonia/disease progression). One death due to study drug toxicity (vanishing bile duct syndrome) was also documented.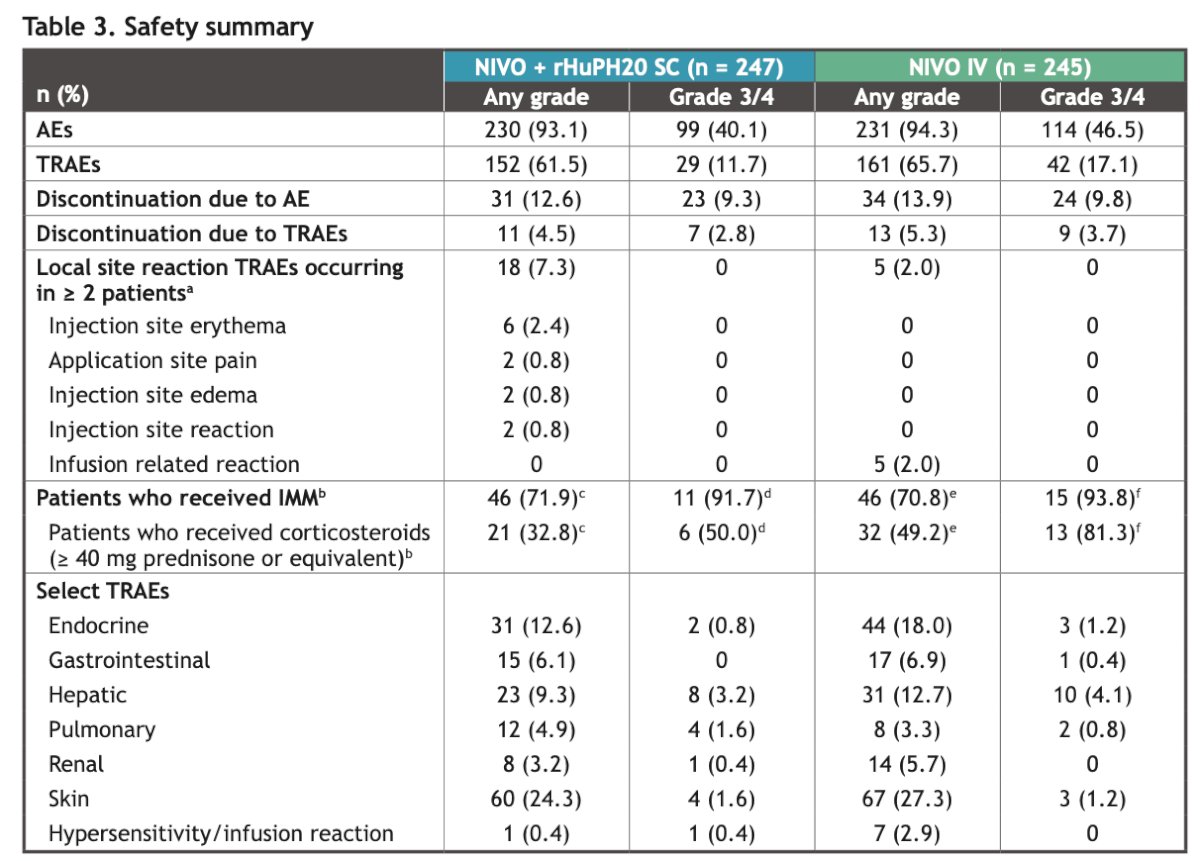
Lastly, the incidence of anti-Nivolumab antibodies was higher with Nivolumab SC (24% vs. 6.9% with Nivolumab IV). However, there was no apparent clinically meaningful impact of the development of anti-Nivolumab antibodies on pharmacokinetics, efficacy, and safety, which is consistent with findings from the primary analysis.
Dr Albiges concluded her poster presentation with the following remarks:
- CheckMate 67T met its co-primary endpoints and secondary endpoints at the primary analysis, showing noninferiority of Nivolumab SC to Nivolumab IV in terms of ORR, OS, and PFS.
- The ORR with extended 15 months minimum follow-up (Nivolumab SC, 26.6% vs. 20.6% Nivolumab IV) was consistent with the primary analysis.
- The safety profile of Nivolumab SC was consistent with that of IV delivery, with no new safety concerns or signals identified after an extended 15-month follow-up.
- The incidence of anti-Nivolumab antibodies was higher with Nivolumab SC compared to Nivolumab IV after an extended 15-month follow-up. However, there is no apparent clinically meaningful impact on pharmacokinetics, efficacy, or safety.
- These data further support the use of Nivolumab SC as a potential new option to improve patient treatment experience and healthcare efficiency.
- This presentation will be accompanied by a simultaneous publication in the Annals of Oncology Journal, detailing the full results of the study.
Presented by: Laurence Albiges, MD, PhD, Medical Oncologist at Gustave Roussy, Université Paris Saclay, Villejuif, France.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References
- O'Shaughnessy J, Sousa S, Cruz J, Fallowfield L, Auvinen P, Pulido C, Cvetanovic A, Wilks S, Ribeiro L, Burotto M, Klingbiel D, Messeri D, Alexandrou A, Trask P, Fredriksson J, Machackova Z, Stamatovic L; PHranceSCa study group. Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): A randomized, open-label phase II study. Eur J Cancer. 2021 Jul;152:223-232. doi: 10.1016/j.ejca.2021.03.047. Epub 2021 Jun 16. PMID: 34147014.
- Locke KW, Maneval DC, LaBarre MJ. ENHANZE® drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 2019 Dec;26(1):98-106. doi: 10.1080/10717544.2018.1551442. Erratum in: Drug Deliv. 2019 Dec;26(1):1300. doi: 10.1080/10717544.2019.1594569. PMID: 30744432; PMCID: PMC6394283.
- Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006 Aug 28;114(2):230-41. doi: 10.1016/j.jconrel.2006.05.027. Epub 2006 Jun 7. PMID: 16876899.
- Saby George et al. Subcutaneous nivolumab (NIVO SC) vs intravenous nivolumab (NIVO IV) in patients with previously treated advanced or metastatic clear cell renal cell carcinoma (ccRCC): Pharmacokinetics (PK), efficacy, and safety results from CheckMate 67T. JCO 42, LBA360-LBA360(2024)


