(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session focusing on rare genitourinary cancers. Dr. Pedro Barata discussed the current treatment paradigm of non-clear cell renal cell carcinoma (nccRCC).

Clear cell RCC remains the most common histologic subtype, accounting for 70–80% of all incident RCC cases. The most common nccRCC histologic subtype is papillary RCC, which accounts for 10–15% of all cases.
Confirming the underlying RCC histologic subtype is key for guiding the choice of systemic therapy in patients with locally advanced/metastatic RCC. This is underlined in the 2024 ESMO guidelines, which state the following: “Establishing the underlying subtype (clear-cell versus variant histology) and presence of sarcomatoid or rhabdoid differentiation using established criteria is strongly recommended due to prognostic and therapeutic implications.” Tissue confirmation can be obtained via examination of the nephrectomy surgical specimen or needle core biopsy sampling of the primary renal tumor or metastatic site(s).
Many groups have demonstrated that RCC histologic subtypes have unique molecular and genomic alterations that drive divergent gene expression profiles, and which have important implications for treatment sensitivity and selection. For example, ccRCC tumors have an increased angiogenesis signature. Conversely, nccRCC tumors have increased cell cycle, fatty acid oxidation/AMPK signaling, and fatty acid synthesis/pentose phosphate signaling scores.1
In 2022, the World Health Organization (WHO) updated the classification of RCCs. The major change is the grouping of these renal tumors, whereby RCC tumors are now classified as either:
- Clear cell renal tumors
- Papillary renal tumors
- Oncocytic and chromophobe renal tumors
- Collecting duct tumors
- Other renal tumors
- Molecularly defined renal carcinomas

Dr. Barata noted that it is very important to recognize that some of these tumors are a ‘manifestation’ of known genetic familial syndromes that are associated with other medical conditions.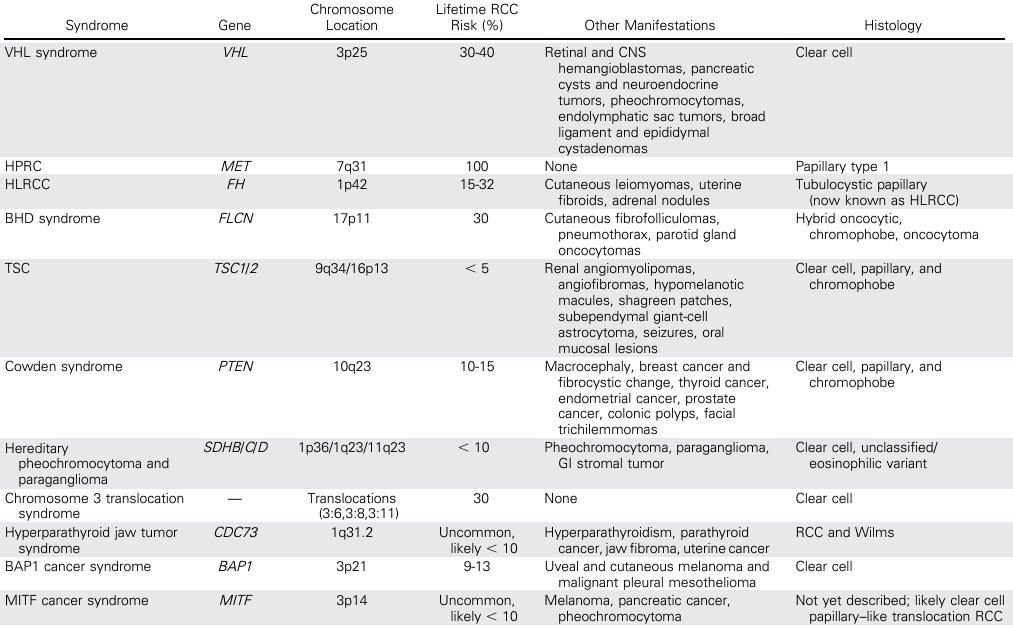
Despite the known hereditary etiology of these tumors, knowing who, what, when, and how genetic testing should be performed remains less clear. A 2021 Delphi consensus of 33 multi-disciplinary experts was published by Bratslavsky et al in 2021 and provides some guidance on this subject matter, with 30 consensus statements now available on this topic.2
Next, Dr. Barata moved on to discuss the clinical trial data informing treatment options for patients with papillary RCC. Cabozantinib was established as the preferred treatment option for patients with papillary RCC based on the results of the PAPMET phase II trial. This was initially a four-arm trial, whereby 152 patients were to be randomly assigned to one of four treatment arms: sunitinib, cabozantinib, crizotinib, or savolitinib. However, a pre-specified futility analysis led to suspension of recruitment to the savolitinib and crizotinib arms, thus effectively making this a sunitinib versus cabozantinib trial. Patients in the cabozantinib group had superior progression-free survival (median 9 versus 5.6 months; hazard ratio [HR]: 0.60, p=0.019). The response rate for cabozantinib was 23% versus 4% for sunitinib (two-sided p=0.010).3 Mature overall survival data is not yet available, but Dr. Barata noted that an overall survival update from this trial is forthcoming (to be published in The Journal of Clinical Oncology).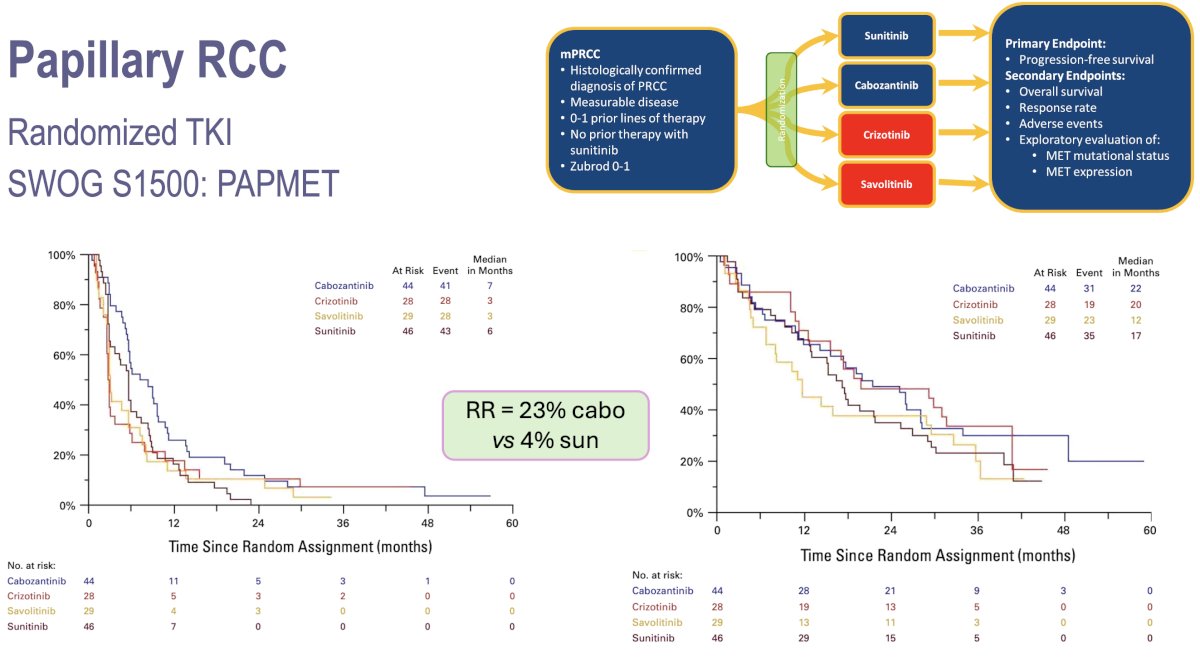
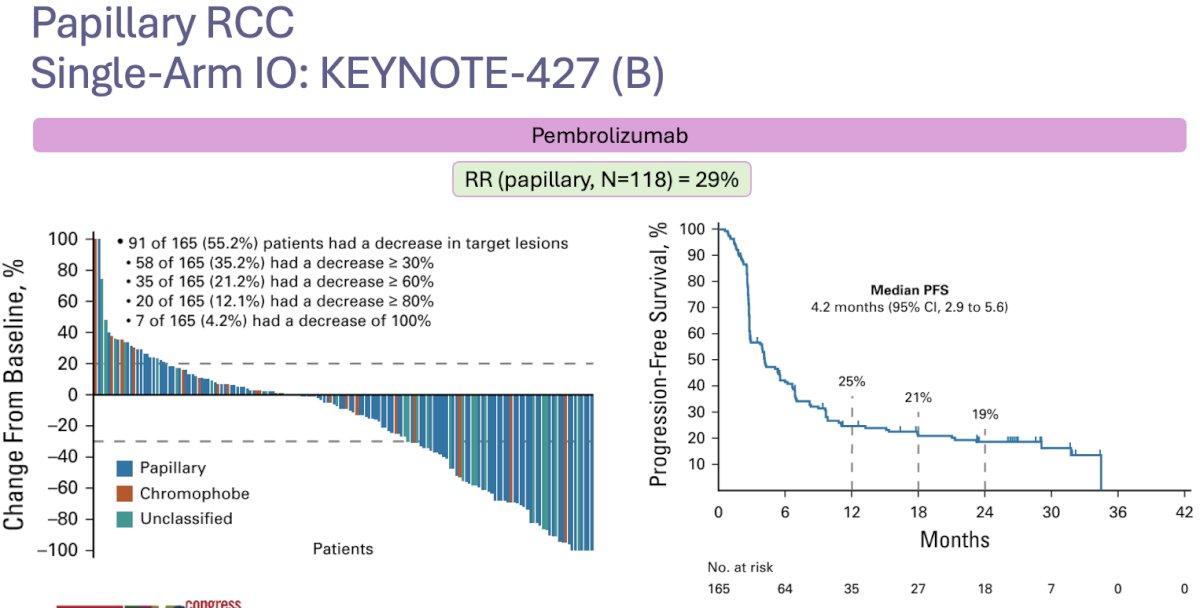
The phase II KEYNOTE-427 trial (cohort B) evaluated the efficacy and safety of single-agent pembrolizumab (anti-PD-1) in patients with metastatic nccRCC who had received no prior systemic therapy. This trial included 165 patients, of whom 72% had papillary disease. The objective response rate (ORR) in papillary RCC patients was 29% (complete response: 6%). The median progression-free survival was 5.5 months, and the median overall survival was 32 months.4
CheckMate 920 was a multicohort phase IIIb/IV trial of nivolumab + ipilimumab in patients with previously untreated advanced RCC and clinical features mostly excluded from phase III trials. Fifty-two patients with nccRCC were included, of whom 35% had papillary disease. Dr. Barata noted that the efficacy results were modest, with an ORR of 20% and a median time-to-response of 2.8 months. The median progression-free survival was 3.7 months, and the median overall survival was 21.2 months.5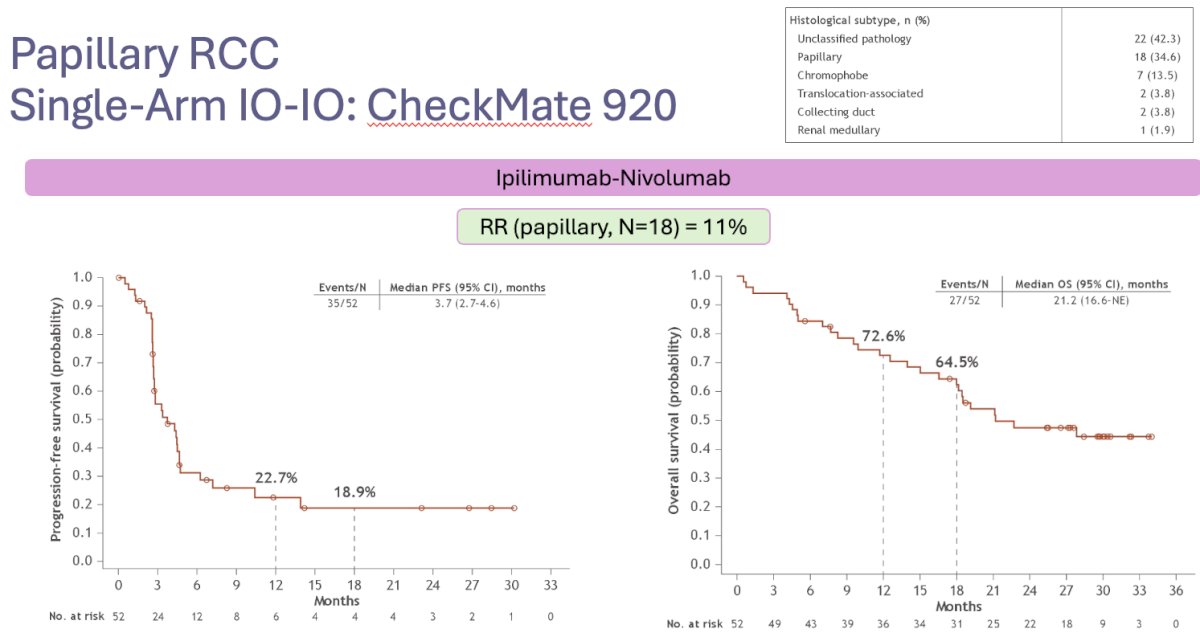
The SUNNIFORECAST trial is comparing the combination of ipilimumab + nivolumab to standard of care (mainly sunitinib) in patients with nccRCC, with the late-breaking results presented by Dr. Lothar Bergmann during this meeting. The study design is illustrated below: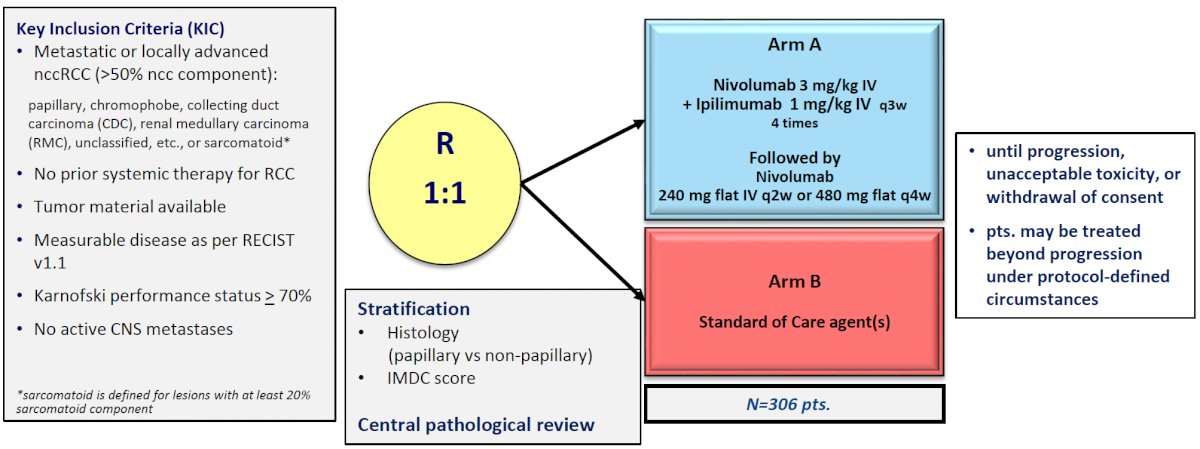
This trial included 309 evaluable patients in the efficacy cohort (papillary: 58%). The median overall survival favored the experimental ipilimumab + nivolumab arm (42 versus 34 months, p=0.3). The 12-month overall survival was significantly higher in the ipilimumab + nivolumab arm: 87% versus 77%. The ORR similarly favored the experimental arm: 29% versus 21%.
There have been further efforts to study other combination regimens of immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs). Prospective single-arm trials of lenvatinib + pembrolizumab (n= 147) and cabozantinib + nivolumab (n= 47) have demonstrated promising ORRs of 49% and 48%, respectively.
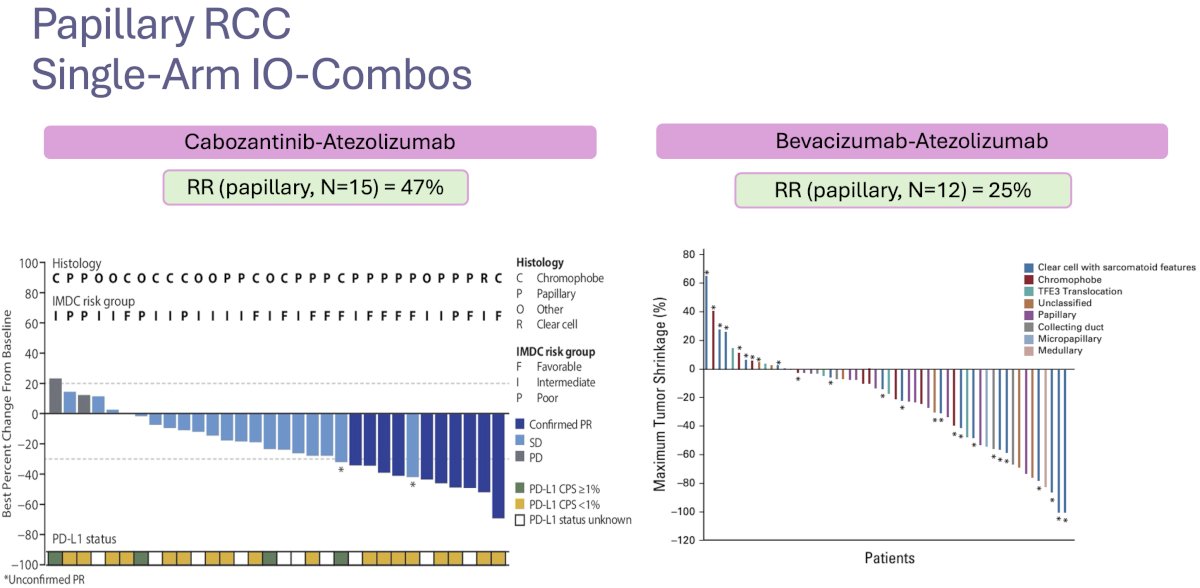
Other single-arm trials of ICI combinations have been published, with the combination of cabozantinib + atezolizumab demonstrating an ORR of 47%, whereas the combination of bevacizumab + atezolizumab demonstrating more limited activity with an ORR of only 25%.8,9
Given that MET gene mutations are a key component of the underlying biology of papillary renal tumors, there has been interest in evaluating savolitinib, a more specific MET inhibitor. Savolitinib monotherapy in MET-positive papillary tumors (CABOSUN trial) has demonstrated limited efficacy, with an ORR of 18%.10 However, the combination of savolitinib + durvalumab in the CALYPSO trial has demonstrated an impressive ORR of 53% in patients with MET-positive papillary RCCs.11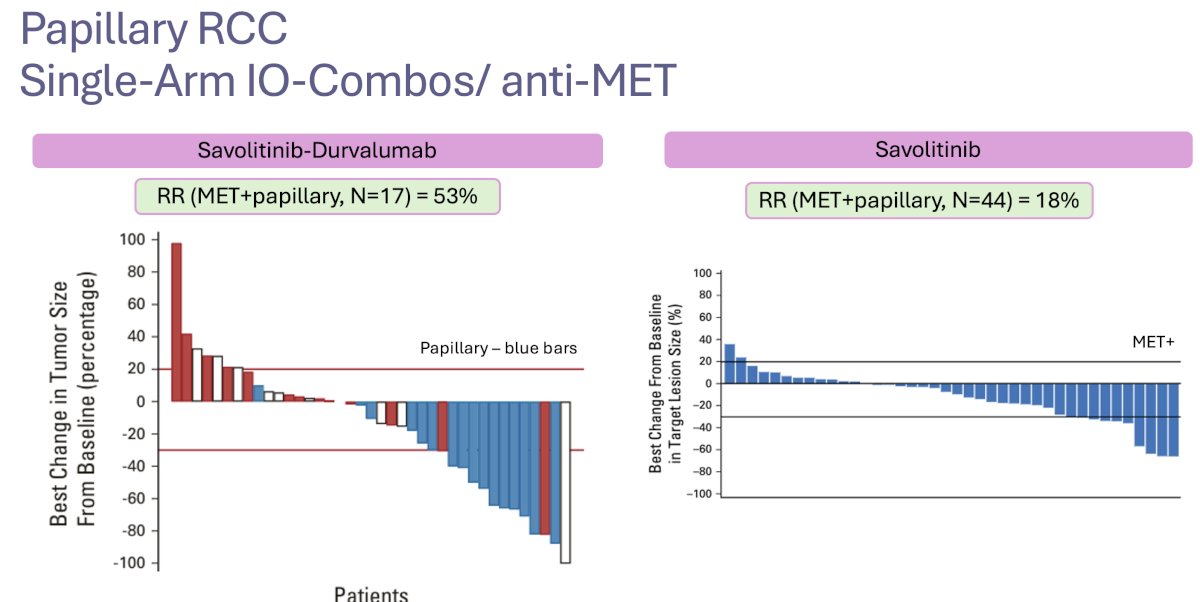
Building upon the results of the phase III COSMIC 313 trial, the combination of cabozantinib + ipilimumab/nivolumab is being evaluated in metastatic RCC patients with variant histology. In the 20 patients with papillary RCC, the ORR was 25%. Updated results of this trial are being presented during this meeting.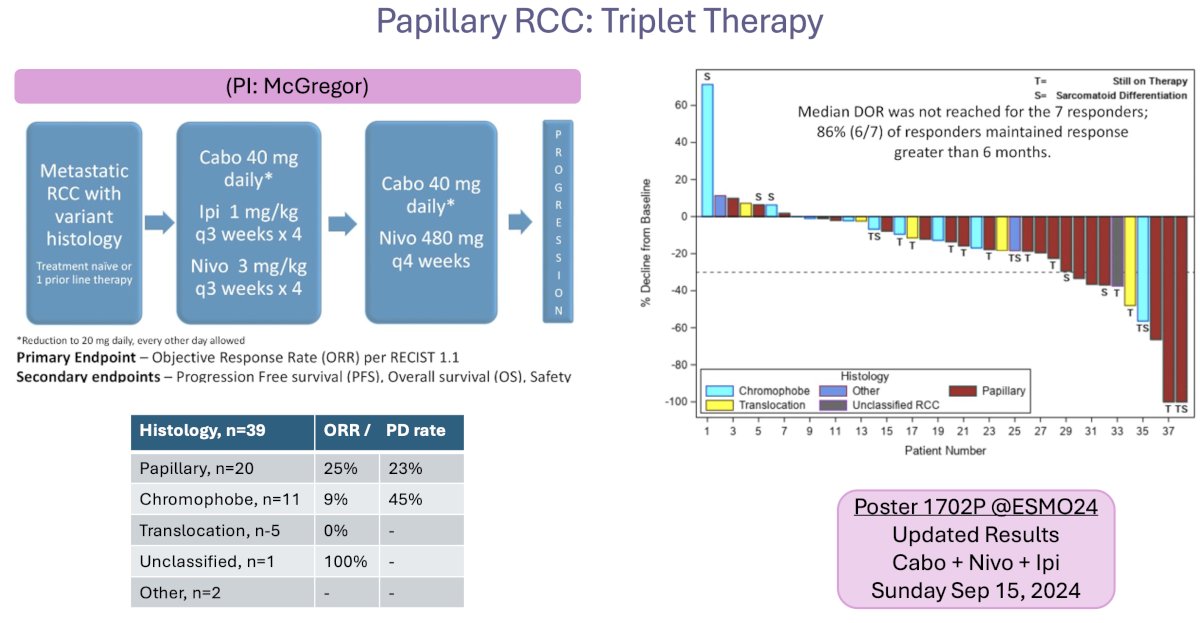
Dr. Barata summarized the current state of the evidence in the papillary RCC disease space by emphasizing that cabozantinib is the preferred treatment option in the 1st line setting. However, no overall survival benefits have been observed to date, with efficacy results limited to ORR and progression-free survival signals.
There are numerous ongoing trials in the papillary RCC disease space, as summarized by Dr. Barata in the slide below:
Next, Dr. Barata discussed the evidence for and treatment options for other nccRCC histologic subtypes. Patients with hereditary leiomyomatosis have fumarate hydratase (FH) gene mutations and typically present with aggressive disease, although this remains a rare condition. The combination of bevacizumab + erlotinib demonstrates very promising efficacy results in this population, with response rates of 72%.12 This combination is being further ‘built upon’ with the triplet therapy combination of bevacizumab + erlotinib + atezolizumab being evaluated.
What about RCC associated with tuberous sclerosis complex? These tumors are secondary to TSC1 and/or TSC2 tumor suppressor gene mutations and are associated with frequent activations of the mTOR pathway. Angiomyolipomas and cysts are the most common renal manifestations, but this syndrome is also associated with papillary, clear cell, and chromophobe renal tumors. Cohort analyses have demonstrated that such patients treated with rapalogs (temsirolimus; everolimus) are more likely to respond if they harbor mutations in mTOR, TSC1, or TSC2. Mutations in TSC1 or TSC2 alone were more common in responders (21%) versus non-responders (6%; p=0.05). Furthermore, 5/12 (42%) subjects with partial responses had mutations in mTOR, TSC1, or TSC2, compared with 4/36 (11%) of non-responders (p=0.03).13

Translocation RCCs account for 1–5% of cases, and these tumors usually grow slowly. Retrospective series have demonstrated the efficacy of both dual ICI and ICI + TKI regimens: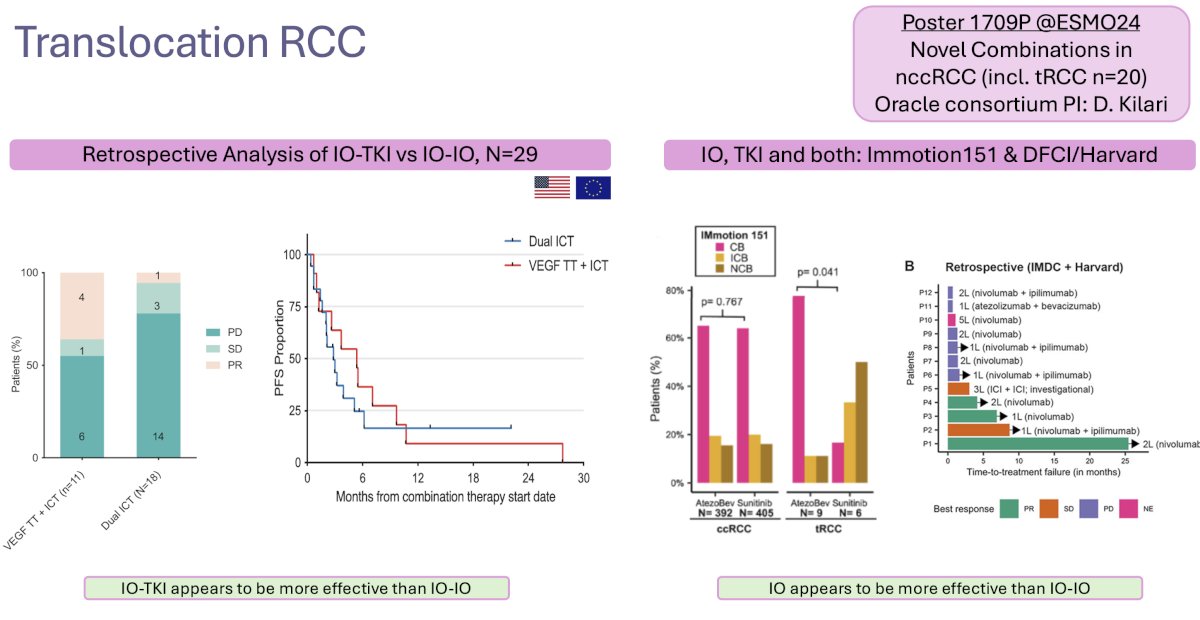
Chromophobe RCC is the 3rd most common nccRCC histologic subtype, accounting for ~5% of cases. Currently, sunitinib, pazopanib, lenvatinib + everolimus, and lenvatinib + pembrolizumab may be used for advanced chromophobe RCC. Highlighted below is the limited trial data available for the treatment of chromophobe RCC patients: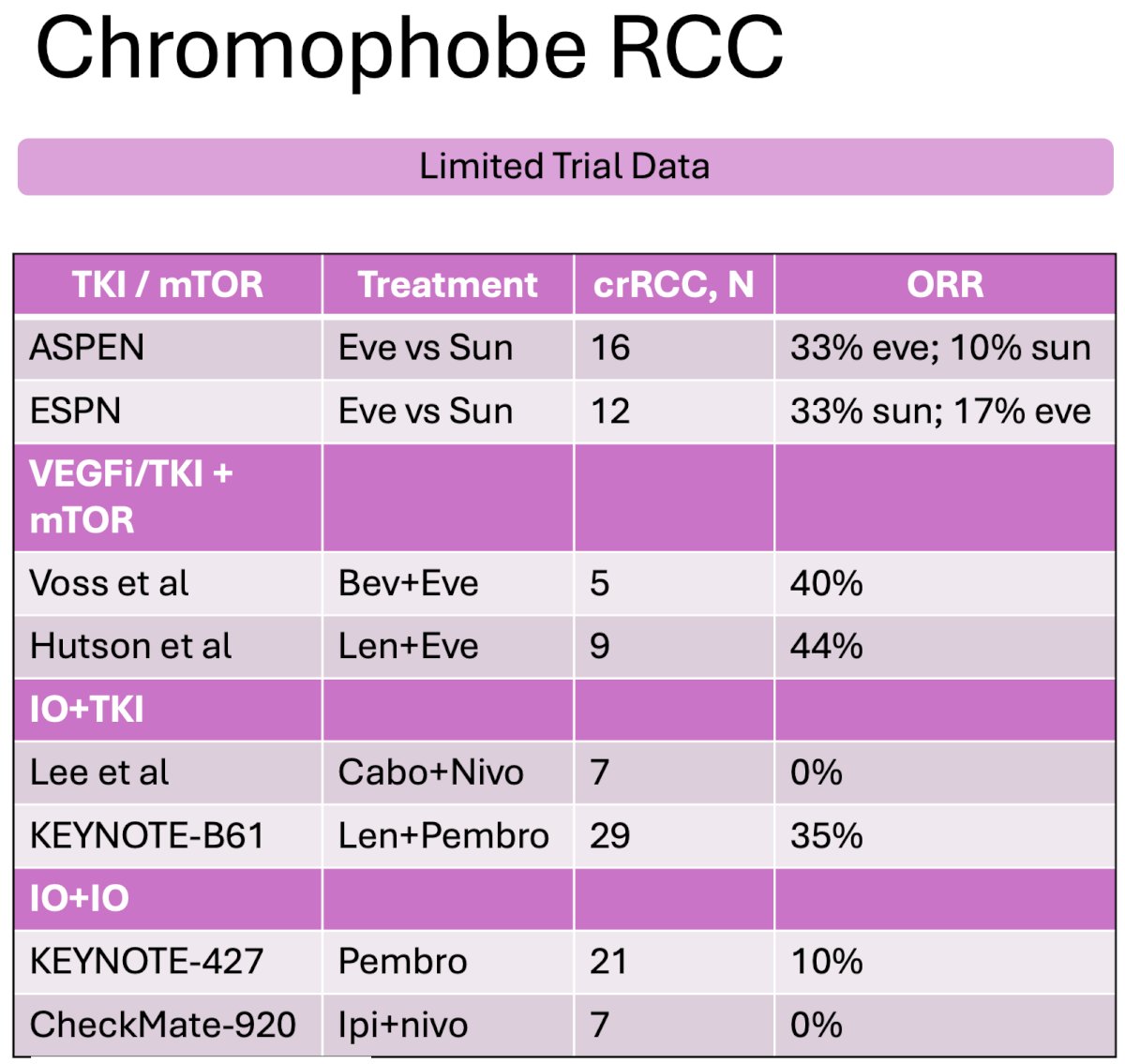
For collecting duct RCCs, an aggressive variant of nccRCCs accounting for ~1% of such tumors, the standard of care remains chemotherapy. Oudard and colleagues demonstrated a response rate of 26% and progression-free survival of 7 months with combination platinum + gemcitabine.14 More recently, cabozantinib was evaluated in 25 such patients and demonstrated a response rate of 35%, with a median progression-free survival of 5 months.15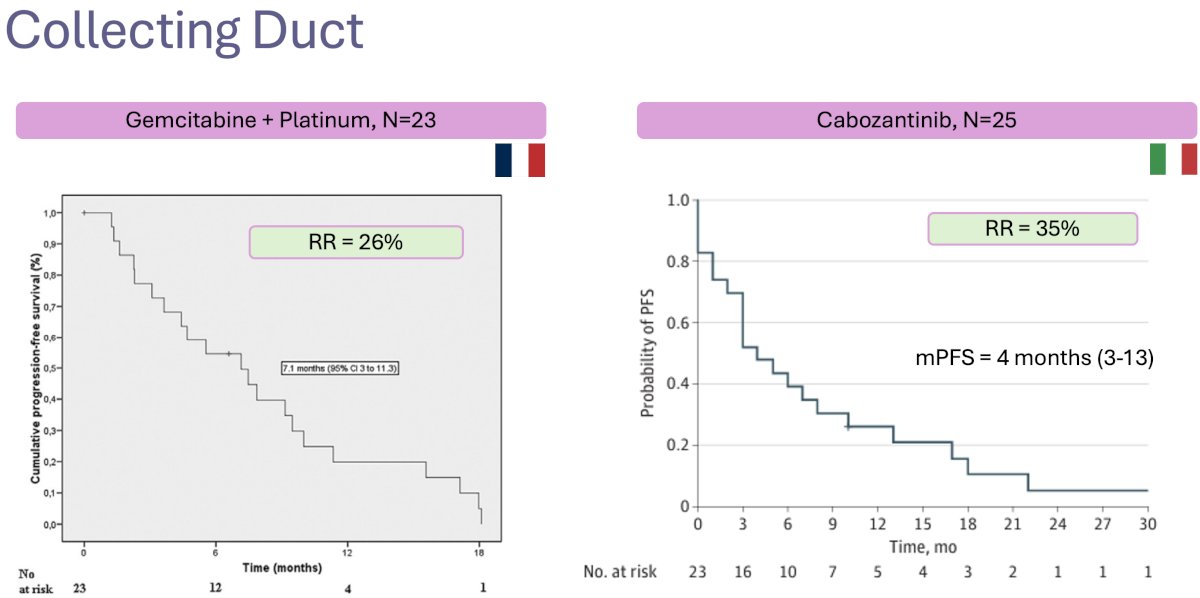
Renal medullary cancer is another rare, aggressive variant that has a known association with sickle cell trait and African American ancestry. Published series from MD Anderson Cancer Center have evaluated the combinations of gemcitabine + doxorubicin and bevacizumab + erlotinib with limited efficacy outcomes.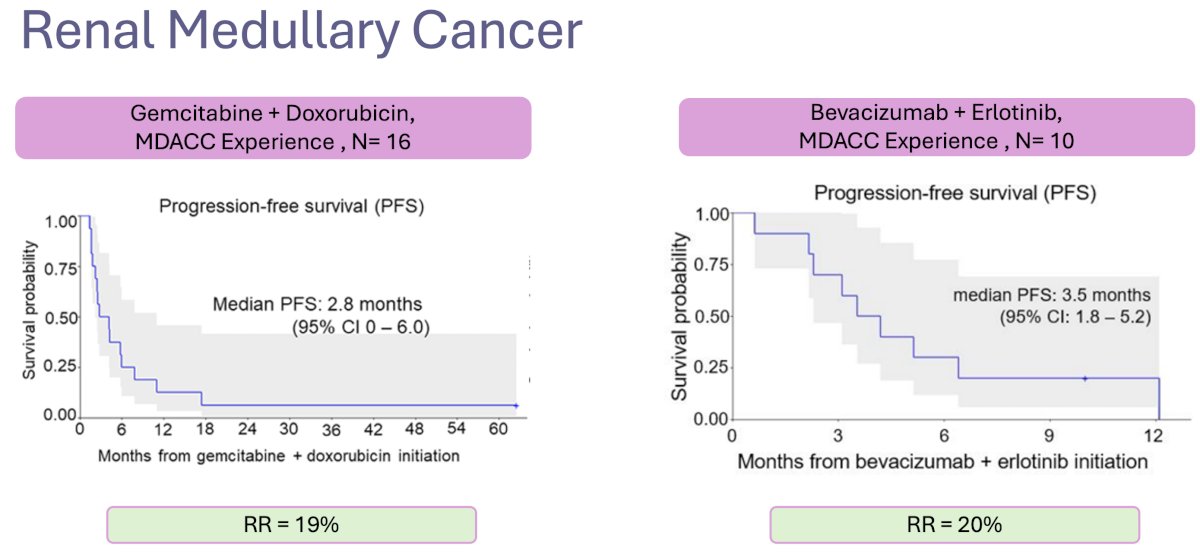
Based on the work of Drs. Tannir and Msaouel from the MDACC, the following is a proposed treatment algorithm for patients with renal medullary cancers:
Dr. Barata concluded as follows:
- The improved molecular characterization of renal tumors has translated into new molecularly-defined RCC subgroups
- More robust (i.e., randomized) data are available in the papillary RCC disease space
- Prospective single-arm studies in rare histologies have been successfully conducted, which is a credit to RCC community groups around the globe.
- Multiple clinical trials are ongoing – take advantage of the resources around you and the medical community’s support is needed.
Presented by: Pedro C. Barata, MD, MSc, Associate Professor at University Hospitals Seidman Cancer Center, Cleveland, OH
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Barata P, Gulati S, Elliott A, et al. Renal cell carcinoma histologic subtypes exhibit distinct transcriptional profiles. J Clin Invest. 2024; 134(11):e178915.
- Bratslavsky G, Mendhiratta N, Daneshvar M, et al. Genetic risk assessment for hereditary renal cell carcinoma: Clinical consensus statement. Cancer. 2021; 127(21):3957-66.
- Pal SK, Tangen C, Thompson Jr IM, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021; 397(10275):695-703.
- McDermott DF, Lee J, Ziobro M, et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2021; 39(9):1029-39.
- Tykodi SS, Gordan LN, Alter RS, et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer. 2022; 10(2):e003844.
- Lee CH, Voss MH, Carlo MI, et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J Clin Oncol. 2022; 40(21):2333-41.
- Albiges L, Gurney H, Atduev V, et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2023; 24(8):881-91.
- McGregor BA, Huang J, Xie W, et al. Phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh). J Clin Oncol. 2023; 41:Number 16_suppl.
- McGregor BA, McKay RR, Braun DA, et al. Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for Patients With Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features. J Clin Oncol. 2020l 38(1):63-70.
- Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017; 35(6):591-7.
- Suarez C, Larkin JMG, Patel P, et al. Phase II Study Investigating the Safety and Efficacy of Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer (CALYPSO). J Clin Oncol. 2023; 41(14):2493-502.
- Srinivasan R, Gurram S, Al Harthy M, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol. 2020; 38:Number 15_suppl.
- Kwiatkowski DJ, Choueiri TK, Fay AP, et al. Mutations in TSC1, TSC2, and MTOR Are Associated with Response to Rapalogs in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016; 22(10):2445-52.
- Oudard S, Banu E, Vieillefond A, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol. 2007; 177(5):1698-702.
- Procopio G, Sepe P, Claps M, et al. Cabozantinib as First-line Treatment in Patients With Metastatic Collecting Duct Renal Cell Carcinoma. JAMA Oncol. 2022; 8(6):910-3.
Related Content: Final Overall Survival Analysis of S1500: A Randomized, Phase II Study Comparing Sunitinib With Cabozantinib, Crizotinib, and Savolitinib in Advanced Papillary Renal Cell Carcinoma


