(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Mini oral session: GU tumours, non-prostate. Dr. Camillo Guglielmo Porta reviewed and discussed the presentations of Drs. Sheng, Ciccarese and Braun.
Dr. Porta discussed the presentations from Drs. Sheng, Ciccarese and Braun. Dr. Sheng presented ETER100 a randomized, open-label, phase Ill study of Anlotinib combined with anti-PD-L1 antibody Benmelstobart (TQB2450) versus Sunitinib in first-line treatment of advanced renal cell carcinoma (RCC). Dr Ciccarese presented the phase 2 TACITO trial exploring Fecal microbiota transplantation (FMT) versus placebo in patients receiving Pembrolizumab plus Axitinib for metastatic renal cell carcinoma. Lastly, Dr Braun presented a post-hoc analysis of CheckMate 9ER exploring novel serum glycoproteomic biomarkers to predict response to Nivolumab plus Cabozantinib versus Sunitinib in advanced RCC.
The goal of this trial was to evaluate Benmelstobart in combination with Anlotinib as a novel ICI (PD-L1) and VEGFR-TKI combination for first-line treatment of mRCC. Dr. Porta summarized the results by showing that PFS favored the combination over VEGFR-TKI monotherapy: the median PFS was 18.96 months compared to 9.76 months (HR = 0.53, 95% CI = 0.42-0.67, p < 0.0001). However, this data is preliminary, and it is still too early to assess OS. He also noted that the antitumor activity was relatively low, with only 1.1% of complete responses documented, and the safety profile was as expected for first-line mRCC treatment.
Compared to other trials in the first-line setting of mRCC, ETER100 has a very short follow-up period (median follow-up of 18.6 months), and only early OS data available. The distribution of patients among the three IMDC risk groups is well-balanced. PFS is similar to other combinations, and despite a good overall response rate, the complete response rate is very low (1.1%) compared to other trials. A comparison across these trials is illustrated in the table below:
Dr. Porta questioned whether this new combination of ICI and VEGFR-TKI is merely another iteration or if it offers something distinct. Anlotinib should be considered a third-generation VEGFR-TKI, similar to Axitinib or Tivozanib, with its primary targets being VEGFR2 and VEGFR3. An advantage of Anlotinib is its high selectivity for VEGFRs, which may result in fewer off-target treatment-related adverse events (TRAEs). Additionally, Anlotinib has an extremely long half-life (96 hours), which could be beneficial for optimizing treatment schedules and maintaining angiogenesis inhibition over time.
Benmelstobart is a humanized anti-PD-L1 IgG1k monoclonal antibody (MoAb) with significant sequence divergence in its complementarity-determining regions compared to benchmark anti-PD-L1 antibodies such as Atezolizumab, Durvalumab, and Avelumab, and it has no Fc activity. As an IgG1k MoAb, its complement-mediated cytotoxicity may contribute to its antitumor activity. Additionally, being a humanized MoAb with less than 3% murine component, it has a low potential for inducing neutralizing antibodies. Furthermore, its equilibrium dissociation constant (KD) performs better compared to other anti-PD-L1 antibodies, as illustrated below: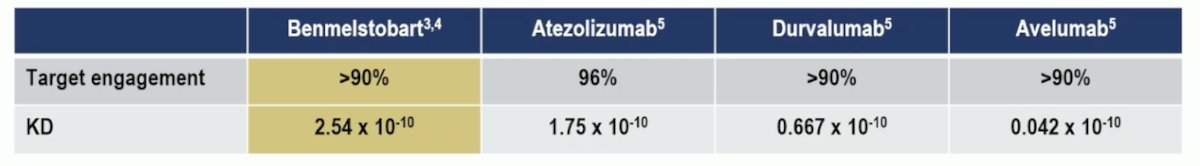
Dr. Porta concluded his discussion of the ETER100 trial by noting that, as a clinician, you might consider using this combination in everyday practice if you believe that selectively inhibiting VEGFRs (and not other kinases) is crucial. Consequently, Anlotinib could become one of your preferred TKIs, especially given its 96-hour half-life. Benmelstobart exhibits pharmacokinetic and structural properties that position it favorably among PD-L1 inhibitors. However, Dr. Porta personally prefers not to opt for an anti-PD-L1 antibody-based combination in mRCC when anti-PD-1 options are available. He also mentioned that market price and global distribution could significantly influence the adoption of this combination, as "money rules the world."
The aim of this trial was to investigate, within a small Phase 2 RCT (n=50), whether FMT from an immunotherapy-responding patient could enhance the antitumor activity of an immune-based combination in treatment-naïve mRCC patients. Dr. Porta summarized the results presented by Dr. Ciccarese, noting that the 1-year PFS rate was increased by 31.7% in patients receiving FMT compared to those receiving placebo, which was statistically significant (p < 0.036). At a median follow-up of 28.2 months, the median PFS was 14.2 months for patients receiving FMT versus 9.2 months for those receiving placebo. Additionally, the overall response rate (ORR) was higher in the FMT group (66.7%), though no complete responses (CRs) were observed in either group. Overall survival (OS) data remain immature as this is a preliminary report.
Early studies have shown promising results with FMT in melanoma. In these trials, donors have included either patients who experienced complete response (CR) or partial response (PR) while progressing on ICI, or healthy volunteers in the upfront first-line therapy setting. In the first-line setting, the overall response rates have been 65%, compared to 20-30% in patients who were progressing on ICI with metastatic melanoma.1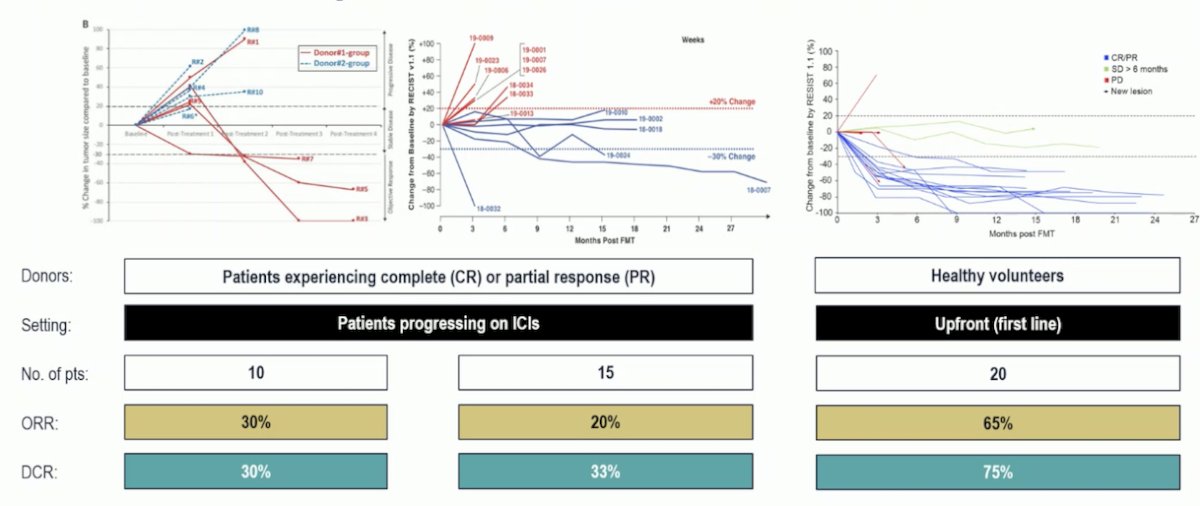
To understand the success of FMT, it’s crucial to identify what’s in the feces. Resident gut bacteria can influence patient responses to cancer immunotherapy. An interesting study by Routy et al. profiled samples from patients with lung and kidney cancers and found that non-responders had low levels of the bacterium Akkermansia muciniphila. Moreover, oral supplementation of this bacterium to antibiotic-treated mice restored their response to immunotherapy. Similarly, another study of melanoma patients receiving PD-1 blockade found a greater abundance of "good" bacteria (such as Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium) in the guts of responding patients, while non-responders had an imbalance in gut flora composition, which correlated with impaired immune cell activity.1,2
Dr. Porta highlighted that, as Dr. Ciccarese noted in her presentation, FMT is challenging to administer. In a small Phase 2 trial, there were numerous obstacles to ensuring that all patients received the three proposed FMT treatments, and achieving this in real-world practice could be even more difficult. Live bacterial supplementation is an alternative to FMT. Two Phase 1 trials have evaluated live bacterial supplements in patients receiving Cabozantinib, Nivolumab, and Nivolumab plus Ipilimumab for mRCC, showing promising results.3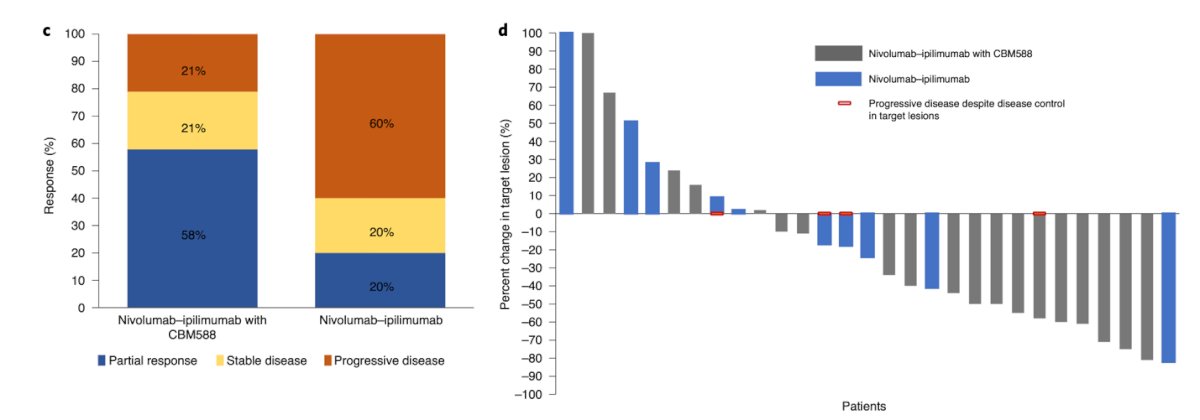
Dr. Porta wrapped the critical appraisal of the TACITO trial with the following conclusions:
- These are intriguing results, but it is too early to apply them in clinical practice, and many key questions remain unanswered.
- The exploitation of gut microbiota to enhance anticancer immune responses is a novel and promising therapeutic strategy, particularly for immunogenic cancers such as melanoma and RCC.
- The optimal approach for boosting anticancer immune responses, whether through FMT or live bacterial supplementation and the best source of feces, still need to be fully determined.
- The bacterial composition of FMT appears to be crucial and should be investigated further, potentially as a stratification factor.
- Larger prospective studies, along with comprehensive immunological and bacteriological analyses, are warranted.
The aim of this post-hoc exploratory analysis of CheckMate 9ER was to investigate the association of glycosylation with response to either Cabozantinib + Nivolumab or Sunitinib. Dr. Porta summarized the results presented by Dr. Braun, who reported that higher sialylation and fucosylation of serum proteins were associated with shorter PFS and OS for both treatments, identifying these as potential prognostic biomarkers. This represents a novel approach to biomarker discovery. Additionally, in patients treated with Cabozantinib + Nivolumab, high levels of CO3 glycopeptide showed a trend towards longer PFS, while low levels of CFAH glycopeptide also trended towards longer PFS.
Glycosylation is a form of co-translational and post-translational modification where a carbohydrate is covalently attached to a target macromolecule, typically a protein. This modification plays a crucial role in the folding and stability of glycoproteins, potentially altering their activity and biological function. Additionally, glycosylation is essential for cell-to-cell adhesion, which may influence cellular immune responses. Given these roles, investigating glycosylation products as potential biomarkers in mRCC is highly relevant.
Dr. Porta noted that despite the excitement surrounding this new data, it must be considered preliminary, as it does not yet meet all the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria. Specifically, it lacks external validation, has not been compared with standard prognostic variables, and has an unproven biological rationale.4
The hypothesis based on these findings is that glycosylation enhances the protein's function by stabilizing it. The increased half-life of CO3 glycopeptide due to sialylation might lead to a pro-inflammatory response, potentially enhancing immunotherapy's antitumor activity. Conversely, low levels of CFAH glycopeptide glycosylation might maintain immune suppressive functions.5
Lastly, Dr. Porta concluded his appraisal with the following remarks about this posthoc analysis of CheckMate 9ER exploring glycoproteomic biomarkers:
- It is too early to translate these results into everyday clinical practice; however, biochemical co- and post-translational modifications are worth studying as potential biomarkers.
- The REMARK and BRISQ criteria should be used to report and validate the proposed glycoproteomic biomarkers.
- Historically, during the cytokine era, complement factors were found to play a prognostic role in mRCC patients treated with IL-2 and IFN, warrants further research.
Presented by: Camillo Guglielmo Porta, Professor, Department of Internal Medicine and Therapeutics, University of Pavia
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:
- Routy B, Lenehan JG, Miller WH Jr, Jamal R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L, Punčochář M, Ernst S, Logan D, Belanger K, Esfahani K, Richard C, Ninkov M, Piccinno G, Armanini F, Pinto F, Krishnamoorthy M, Figueredo R, Thebault P, Takis P, Magrill J, Ramsay L, Derosa L, Marchesi JR, Parvathy SN, Elkrief A, Watson IR, Lapointe R, Segata N, Haeryfar SMM, Mullish BH, Silverman MS, Burton JP, Maleki Vareki S. Author Correction: Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2024 Feb;30(2):604. doi: 10.1038/s41591-023-02650-8. Erratum for: Nat Med. 2023 Aug;29(8):2121-2132. doi: 10.1038/s41591-023-02453-x. PMID: 37923839.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018 Jan 5;359(6371):104-108. doi: 10.1126/science.aao3290. PMID: 29302014; PMCID: PMC6707353.
- Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, Hsu J, Zengin Z, Salgia N, Salgia S, Malhotra J, Chawla N, Chehrazi-Raffle A, Muddasani R, Gillece J, Reining L, Trent J, Takahashi M, Oka K, Higashi S, Kortylewski M, Highlander SK, Pal SK. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022 Apr;28(4):704-712. doi: 10.1038/s41591-022-01694-6. Epub 2022 Feb 28. PMID: 35228755; PMCID: PMC9018425.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005 Aug 22;93(4):387-91. doi: 10.1038/sj.bjc.6602678. PMID: 16106245; PMCID: PMC2361579.
- Daugan MV, Revel M, Thouenon R, Dragon-Durey MA, Robe-Rybkine T, Torset C, Merle NS, Noé R, Verkarre V, Oudard SM, Mejean A, Validire P, Cathelineau X, Sanchez-Salas R, Pickering MC, Cremer I, Mansuet-Lupo A, Alifano M, Sautès-Fridman C, Damotte D, Fridman WH, Roumenina LT. Intracellular Factor H Drives Tumor Progression Independently of the Complement Cascade. Cancer Immunol Res. 2021 Aug;9(8):909-925. doi: 10.1158/2326-6066.CIR-20-0787. Epub 2021 May 26. PMID: 34039652.


