(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Mini oral session: GU tumours, non-prostate. Dr. Xinan Sheng presented the results from the ETER100 phase III randomized, open-label study, exploring Anlotinib combined with anti-PD-L1 antibody Benmelstobart (TQB2450) versus sunitinib in first-line treatment of advanced renal cell carcinoma (RCC).
Approximately 43,400 new cases of RCC were diagnosed worldwide in 2022. To date, localized RCC can be successfully managed with surgery, while metastatic RCC (mRCC) has a poor prognosis with a 5-year survival rate of about 12%. (1) The standard of care for mRCC has become combinations of immune checkpoint inhibitors (anti-PD1 or CTLA-4) and antiangiogenic agents (VEGF-TKI).
Benmelstobart is a humanized monoclonal antibody against PD-L1, approved for the first-line treatment of squamous cell carcinoma of the lung in China. Anlotinib is an anti-angiogenic oral multi-target tyrosine kinase inhibitor (TKI) that has already been approved for multiple solid neoplasms in China. The ETER100 is a randomized, open-label, phase III trial (NCT04523272) aimed at comparing the efficacy and safety of Benmelstobart plus Anlotinib versus sunitinib as first-line treatment for advanced ccRCC patients.
The ETER100 trial included patients with locally advanced or metastatic clear cell renal cell carcinoma (ccRCC) who had not been previously exposed to systemic therapy. Patients had an ECOG performance status (PS) of 0 or 1 and at least one measurable lesion per RECIST v1.1. Participants were randomized to receive either Anlotinib (12 mg QD, D1~D14/Q3w) plus Benmelstobart (1200 mg, D1/Q3w) (n=266) or Sunitinib (50 mg QD, D1-D28/Q6w) (n=265). The primary endpoint of the study was progression-free survival (PFS) as determined by blinded independent central review (BICR). Patients were stratified based on the International Metastatic RCC Database Consortium (IMDC) risk group. The study design schema is shown below:
A total of 687 patients were screened for eligibility, and 531 were randomized. Of these, 264 received study treatment in the Benmelstobart + Anlotinib arm, and 264 in the Sunitinib arm.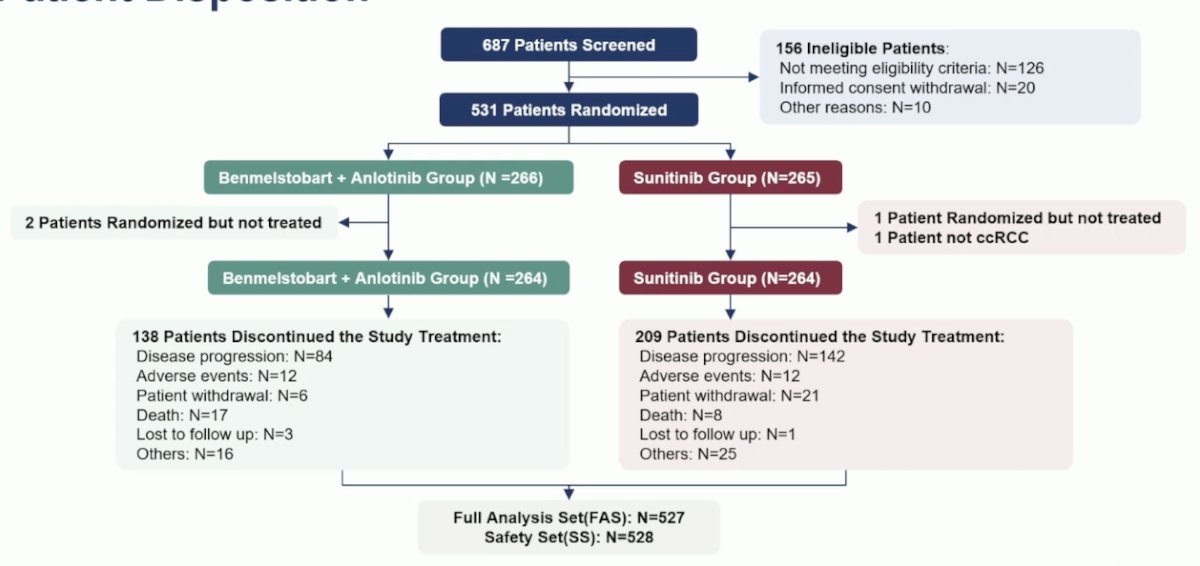
The baseline characteristics were well-balanced and are illustrated in the table below. In the overall population, the median age was 60 years, most patients (76%) were male, most were in the IMDC intermediate-risk group (71.5%), and the most common site of metastasis was the lung (61.6%).
The primary endpoint of PFS by BICR was met at a median follow-up of 18.6 months. The median PFS in the Benmelstobart + Anlotinib group was 18.96 months compared to 9.76 months in the Sunitinib group (HR 0.53, p<0.0001). The subgroup analysis of BICR-assessed PFS was consistent across all subgroups except in patients with sarcomatoid differentiation (n=17) and the favorable IMDC risk group (n=73).
Overall survival was also significantly better in the experimental arm, with the median not reached in both arms and an HR of 0.66 (p=0.0673). The overall response rate was significantly better in the Benmelstobart + Anlotinib arm at 71.6% compared to 25.1% in the Sunitinib arm.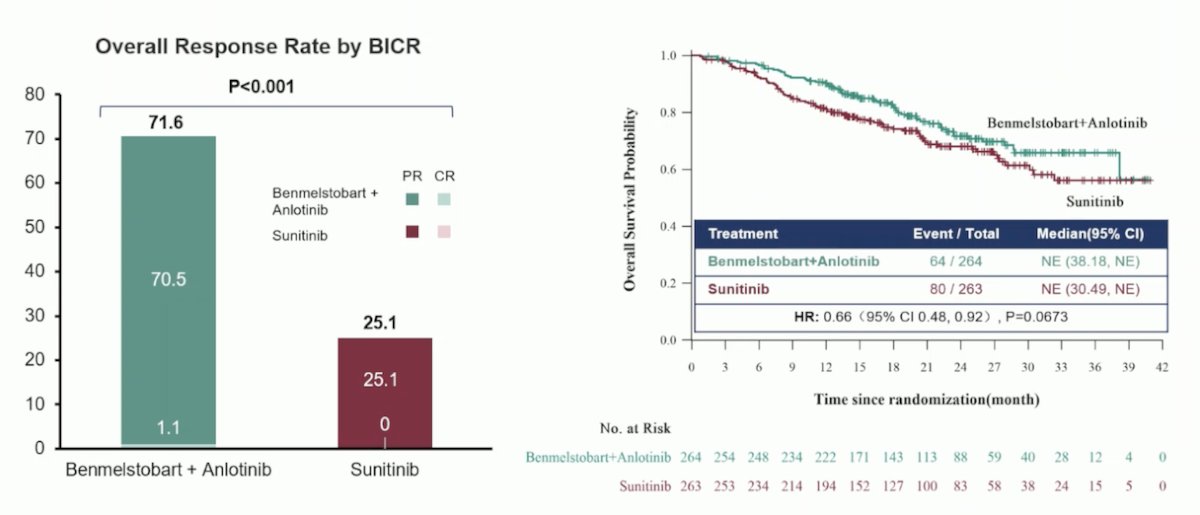
In terms of safety, treatment-emergent adverse events (TEAEs) were similar between both groups, with Grade ≥ 3 TEAEs documented in 75% of the experimental arm and 74.6% of the control arm. The most common treatment-related adverse events (TRAEs) are outlined below: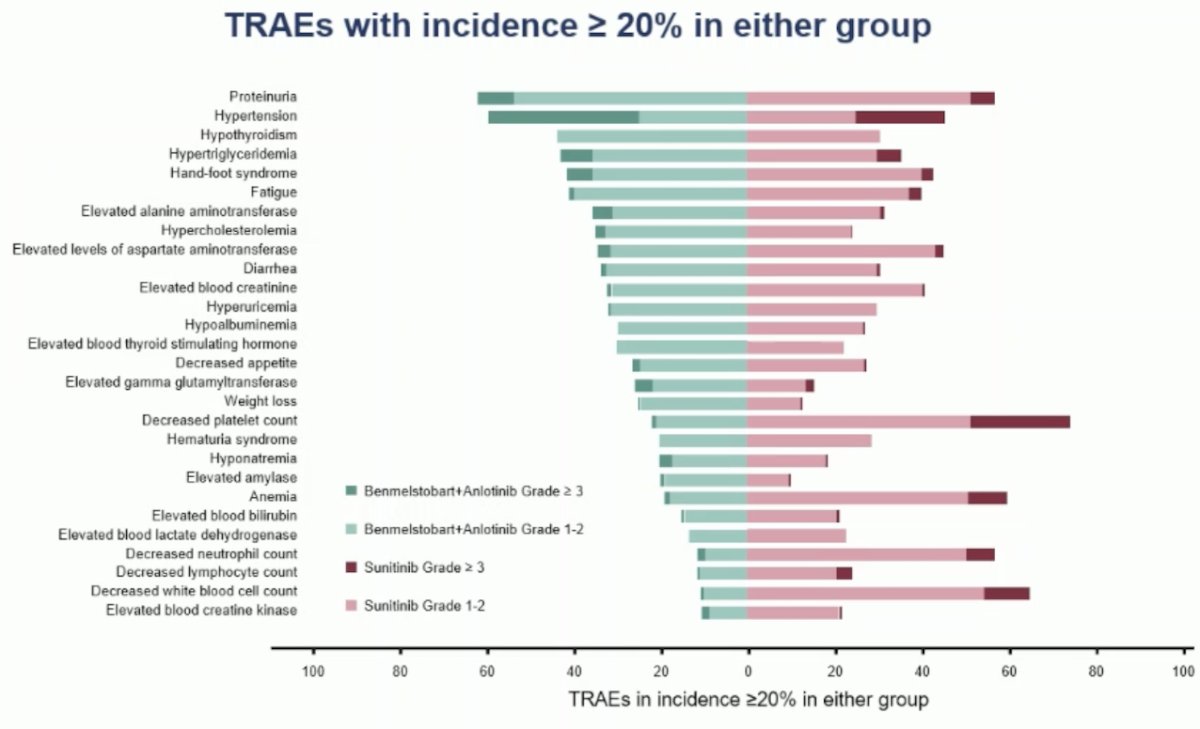
Dr Sheng concluded his presentation with the following remarks:
- Benmelstobart and Anlotinib as first-line treatment for mRCC patients provided superior clinical outcomes compared to sunitinib:
- Significantly improved the median PFS (18.96m vs 9.76m)
- Higher ORR (71.6% vs 25.1%, while OS is still immature.
- The combination of Benmelstobart and Anlotinib was generally well-tolerated, with a safety profile consistent with each individual agent.
- Benmelstobart in combination with Anlotinib has the potential to become a new standard first-line treatment for advanced RCC.
Presented by: Xinan Sheng, MD, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Genitourinary Oncology, Peking University Cancer Hospital & Institute, Beijing, China
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:

