(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Mini oral session: GU tumours, non-prostate. Dr. David A. Braun presented an exploratory analysis from CheckMate 9ER exploring Novel serum glycoproteomic biomarkers to predict response to Nivolumab plus Cabozantinib versus Sunitinib in advanced RCC (aRCC).
Dr. Braun began by saying that in the CheckMate 9ER trial, Nivolumab + Cabozantinib continues to show significant long-term benefits over Sunitinib in progression-free survival (PFS), overall survival (OS), and overall response rate (ORR), with a median follow-up of 55.6 months.1
As predictive biomarkers for aRCC remain limited, gaining deeper insights into the underlying biology driving treatment responses is crucial, and currently predictive biomarkers in aRCC are lacking. Altered protein glycosylation patterns, which are hallmarks of malignant transformation, contribute to sustained cell proliferation, resistance to apoptosis, angiogenesis, and immune evasion. Notably, protein hypersialylation (excessive attachment of sialic acid residues to glycoproteins) has been linked to resistance against PD1-blockade.2
Dr. Braun presented their post-hoc exploratory analysis investigating the association of site-specific glycopeptide modifications with treatment responses to Nivolumab + Cabozantinib and Sunitinib in aRCC, using pre-treatment serum samples. Glycoproteomics analysis was conducted via the InterVenn GlycoVision™ platform, which measures glycopeptides. The study design is illustrated below.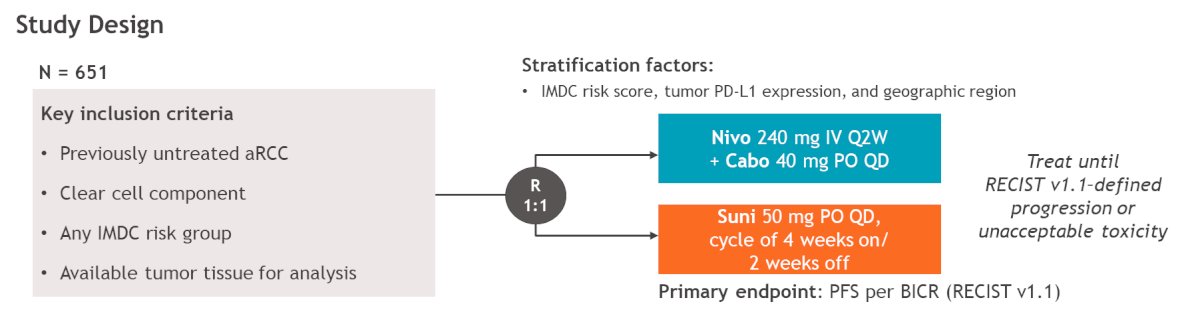
Dr. Braun and colleagues identified a total of 24 serum glycopeptides that were associated with PFS or OS with Nivolumab + Cabozantinib on Cox multivariable analysis. The glycopeptides with significant association with PFS or OS in the Nivolumab + Cabozantinib arm are shown in blue in the figure below.
An interesting finding was that subjects with higher levels of sialylated and fucosylated glycans (n=283) had poorer PFS and OS with both Nivolumab + Cabozantinib and Sunitinib, suggesting a potential role of protein glycosylation in influencing therapeutic response. The sialylated and fucosylated N-glycopeptides are illustrated in the graphic below.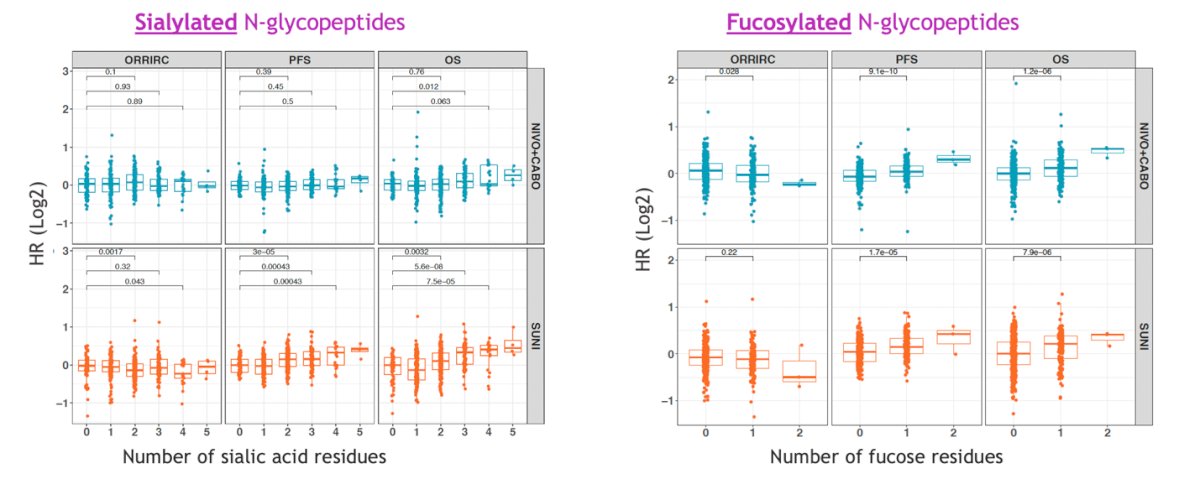
Furthermore, high serum levels of complement protein 3 (CO3) glycopeptide and low serum levels of complement factor H (CFAH) glycopeptide may predict a favorable response to Nivolumab + Cabozantinib versus Sunitinib. Specifically, a trend toward improved PFS was observed with high levels of CO3. Conversely, CFAH glycopeptide may mediate immunosuppression and acts as an innate immunological checkpoint protein, with lower levels associated with a more favorable response.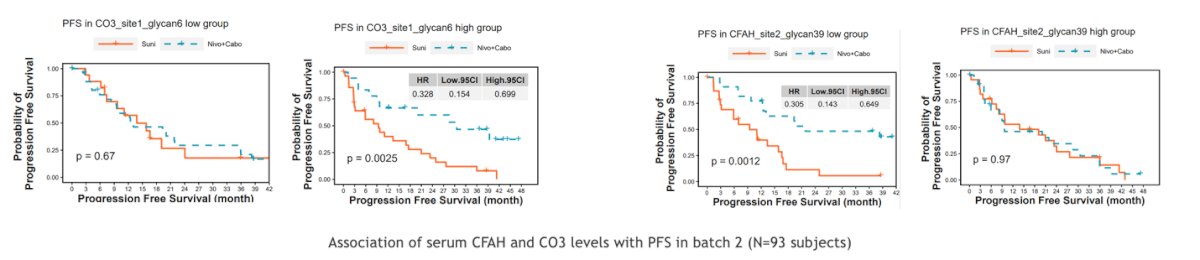
Dr. Braun summarized his presentation with the following messages:
- This post-hoc analysis of the CheckMate 9ER trial, which studied serum site-specific glycopeptides to identify biomarkers associated with the efficacy of Nivolumab + Cabozantinib versus Sunitinib, indicates that higher degrees of fucosylation and sialylation of serum proteins are associated with worse clinical outcomes (PFS, OS) and response to treatment for both Nivolumab + Cabozantinib and Sunitinib.
- Fucosylation and sialylation of serum proteins should be considered negative prognostic factors in aRCC.
- Serum glycoproteins involved in the complement cascade and lipid metabolism (CO3 and CFAH) are potentially predictive of response to the Nivolumab + Cabozantinib combination compared to the TKI alone.
- Following this post-hoc analysis of CheckMate 9ER, there is a pressing need to evaluate the prognostic and predictive potential of these glycopeptide biomarkers in additional clinical trials.
Presented by: David A. Braun, MD, PhD, Assistant Professor of Medicine (Medical Oncology), Department of Medicine, Yale University, New Haven, CT
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
Related content: Glycoproteomics in Kidney Cancer: CheckMate 9ER Biomarker Analysis - David Braun
- Bourlon, Maria Teresa, et al. "Nivolumab plus cabozantinib (N+ C) vs sunitinib (S) for previously untreated advanced renal cell carcinoma (aRCC): Results from 55-month follow-up of the CheckMate 9ER trial." (2024): 362-362.
- Boland, Patrick, et al. "Immunotherapy to treat malignancy in patients with pre-existing autoimmunity." Journal for immunotherapy of cancer 8.1 (2020).


