(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a session summarizing updates in the management of advanced renal cell carcinoma (RCC). Dr. Mir discussed the role of cytoreductive nephrectomy in the immunotherapy era.
Dr. Mir began by highlighting the following:
- All trials of cytoreductive nephrectomy are of patients with synchronous (i.e., de novo) metastatic clear cell RCC
- Existing prospective randomized trials evaluating the role of cytoreductive nephrectomy were performed in the tyrosine kinase inhibitor (TKI) era
- The synergistic effects of medical therapies and surgery are being constantly re-analyzed
The rationale for cytoreductive nephrectomy in metastatic kidney cancer is to reduce the tumor burden, improve symptoms, and potentially enhance the effectiveness of systemic therapies. By removing the primary tumor, cytoreductive nephrectomy may help alleviate local symptoms such as pain or bleeding, and decrease the overall tumor load, which could improve the immune system’s or targeted therapies' ability to control metastatic disease.
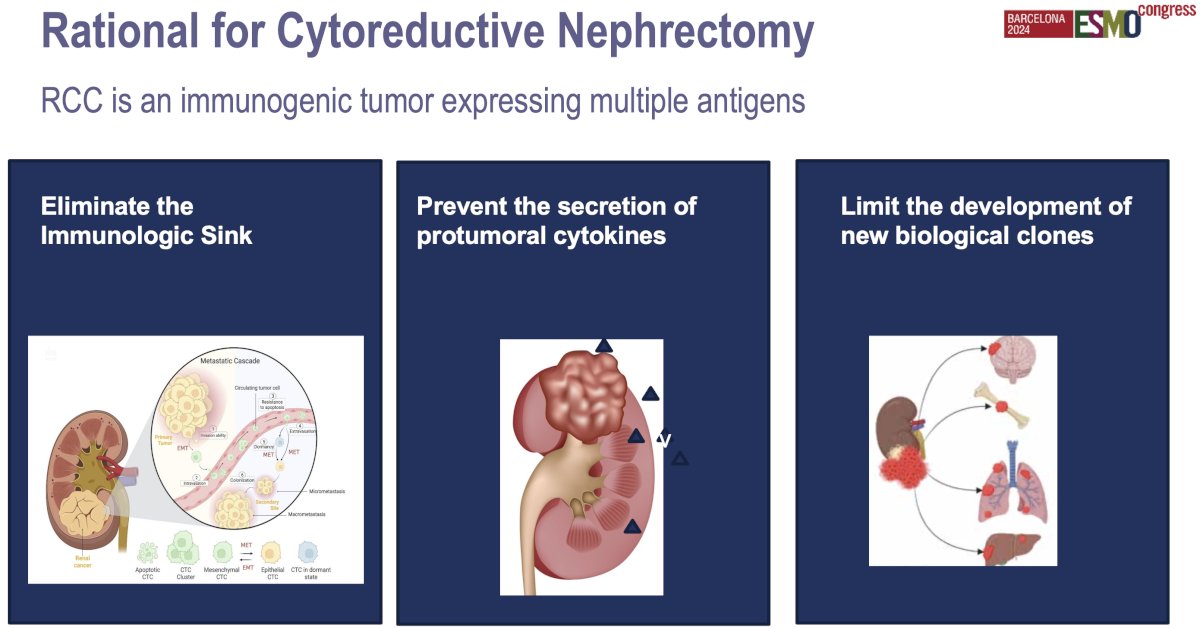
The role of cytoreductive nephrectomy in the era of modern targeted therapies and immune checkpoint inhibitors has evolved. Current guidelines from major organizations, such as the National Comprehensive Cancer Network (NCCN), the European Association of Urology (EAU), and the American Urological Association (AUA), reflect this shift. These guidelines suggest adopting a more selective approach to cytoreductive nephrectomy in metastatic RCC patients, favoring systemic therapy first in most cases and reserving surgery for patients with a good prognosis, low metastatic burden, and good performance status.

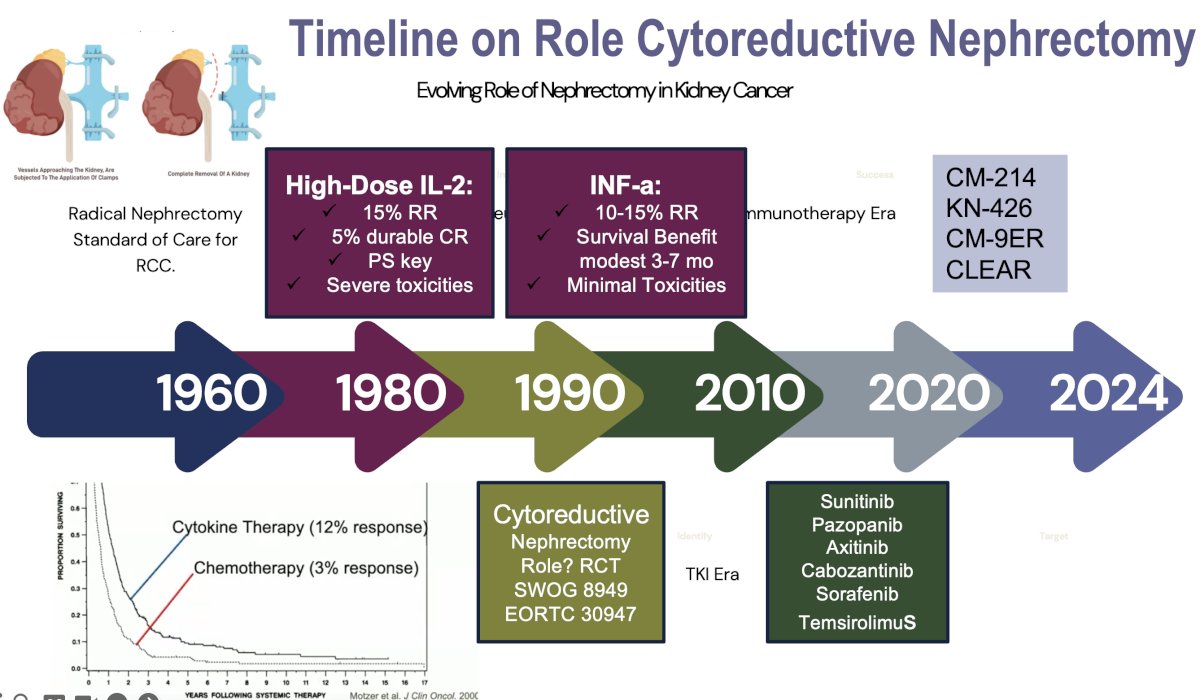
The role of cytoreductive nephrectomy in metastatic RCC has significantly evolved since the cytokine era. In the 1990s and early 2000s during the cytokine era of interferon-alpha and interleukin-2, cytoreductive nephrectomy was widely performed based on the results of trials that demonstrated a survival benefit for cytoreductive nephrectomy.1,2
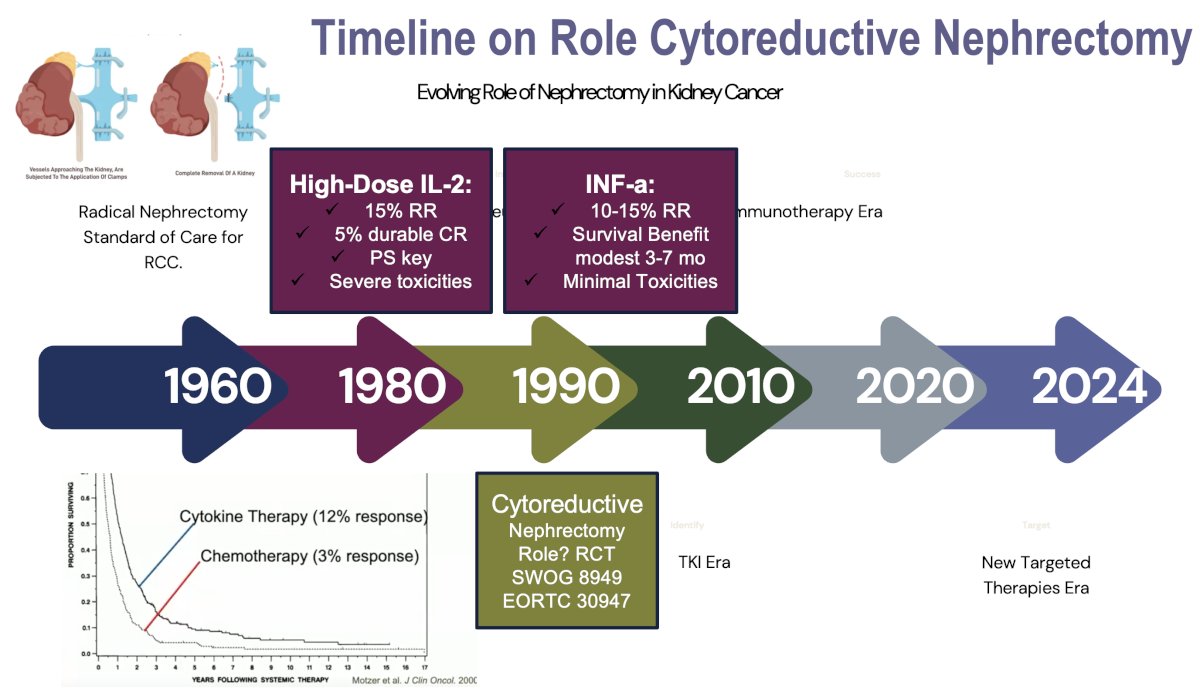
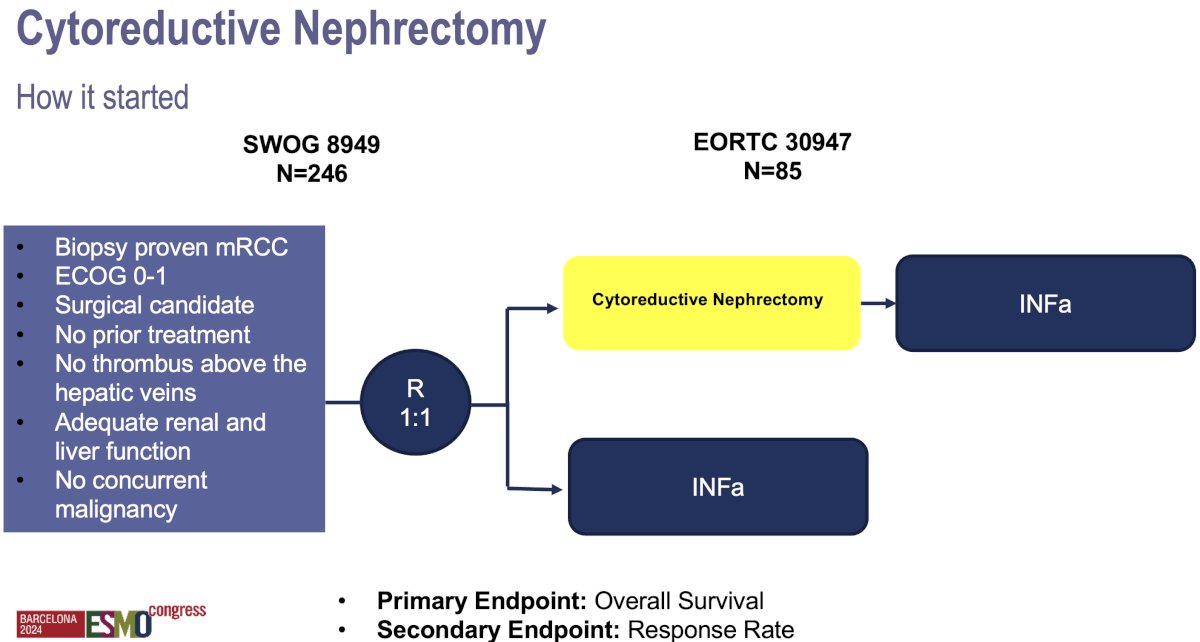
The 1st randomized trial to evaluate the role of cytoreductive nephrectomy was the SWOG 8949 trial, which was soon followed by the EORTC 30947 trial. These trials randomized metastatic RCC patients who had not received prior systemic therapy and had no thrombus above the level of the hepatic veins to cytoreductive nephrectomy followed by interferon-alfa versus immediate interferon-alpha.
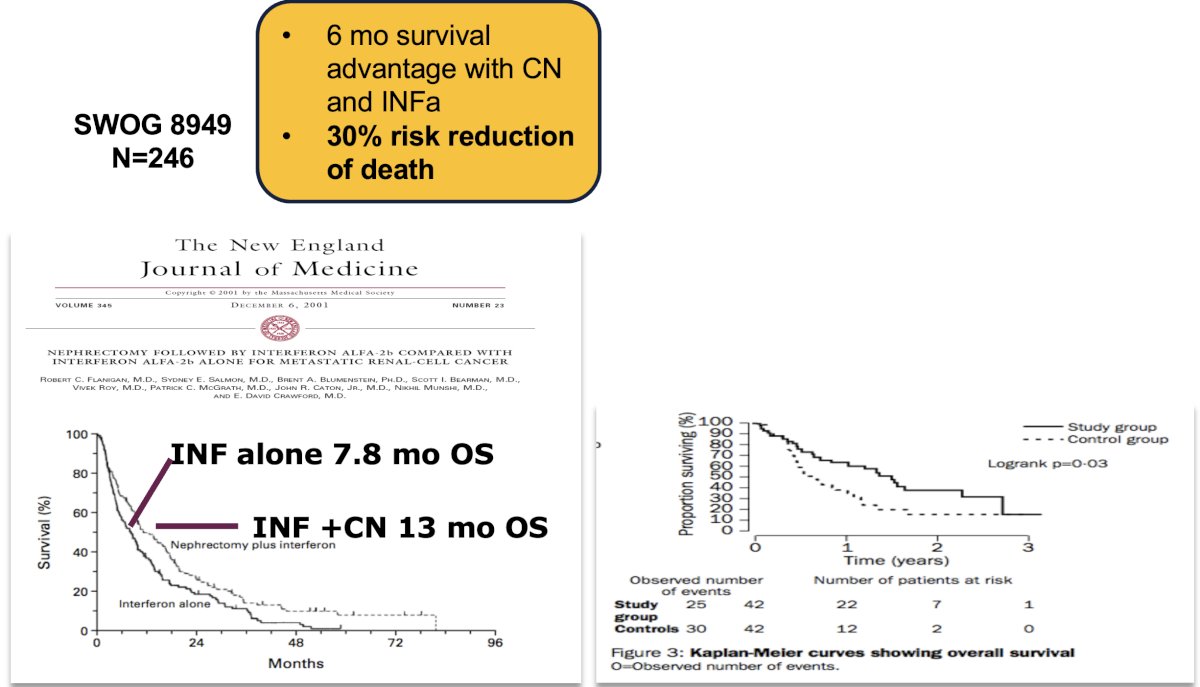
These trials demonstrated an ~30% risk reduction benefit with cytoreductive nephrectomy, resulting in a 6-month survival advantage. The greatest survival benefit was observed in patients with a good performance status and in those with pulmonary-only metastases.
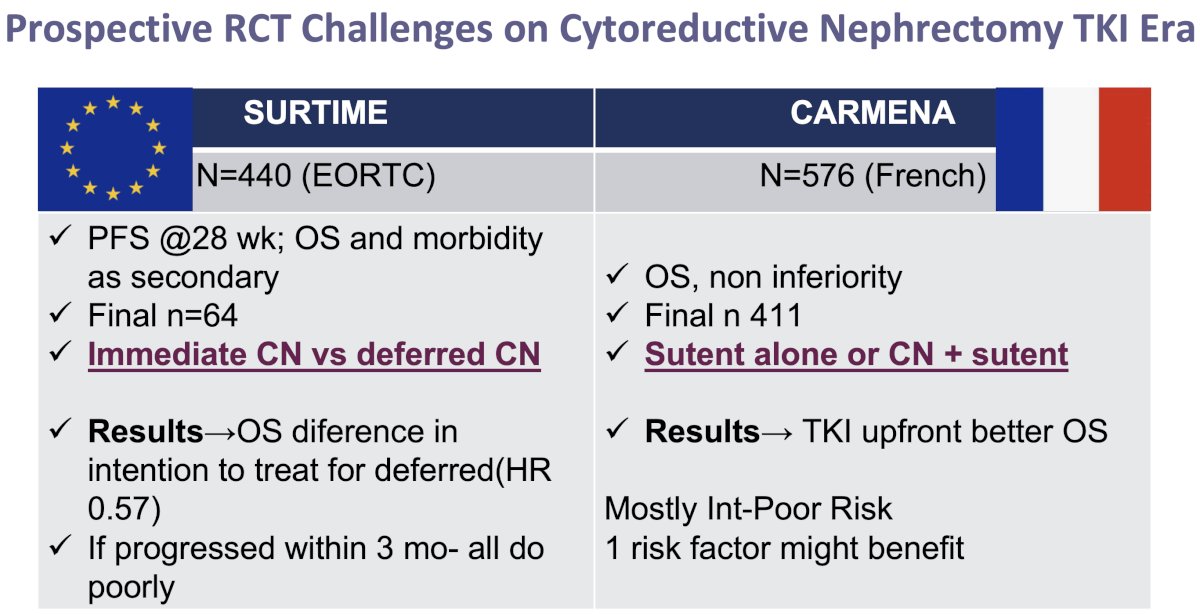
With the advent of targeted therapies in the mid-2000s, particularly TKIs, including sunitinib, the necessity of cytoreductive nephrectomy was brought into question. The CARMENA trial (2018) showed that sunitinib alone was non-inferior to upfront cytoreductive nephrectomy followed by sunitinib, particularly in patients with poor prognostic features.3 This trial shifted the paradigm, suggesting that upfront nephrectomy might not always be necessary, especially for those with a significant metastatic burden. Subsequently, the SURTIME trial (2019) introduced the concept of deferred nephrectomy, demonstrating that performing surgery after a period of systemic therapy could benefit select patients with a favorable response.4
In the current era of immunotherapy, combination regimens using immune checkpoint inhibitors (e.g., nivolumab + ipilimumab) have become established as effective 1st line treatment options, further questioning the role of upfront cytoreductive nephrectomy. Today, cytoreductive nephrectomy is reserved for well-selected patients with good performance status, low metastatic burden, and good initial responses to systemic therapy responsiveness to initial therapy, resulting in a more personalized, selective approach.
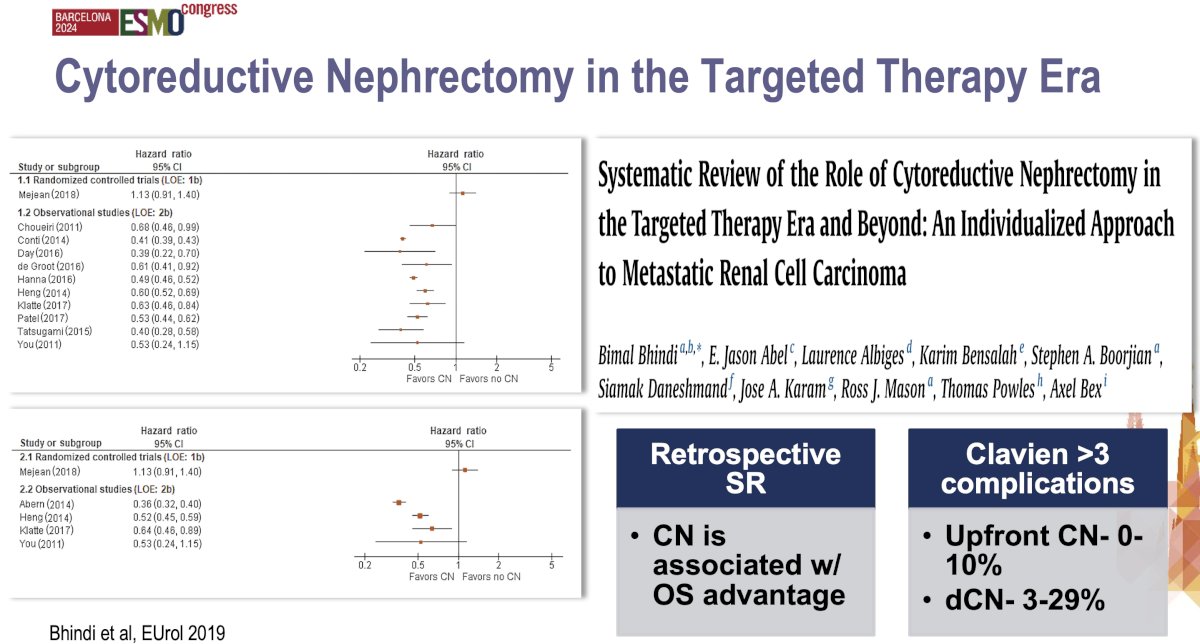
The systematic review by Bhindi et al. published in 2019 in European Urology evaluated the role of cytoreductive nephrectomy within the context of modern systemic therapies. The systematic review demonstrated a 40% overall survival benefit with cytoreductive nephrectomy. However, the authors from this review concluded that cytoreductive nephrectomy should not be routinely performed, particularly in poor-risk patients. It may, however, offer survival benefits for carefully selected patients, especially those with good performance status and a favorable response to systemic therapy. The study emphasized a shift towards a personalized approach, advocating for deferred cytoreductive nephrectomy after initial systemic treatment to assess response prior to considering surgery. This systematic review supports selective, rather than universal, cytoreductive nephrectomy adoption.5
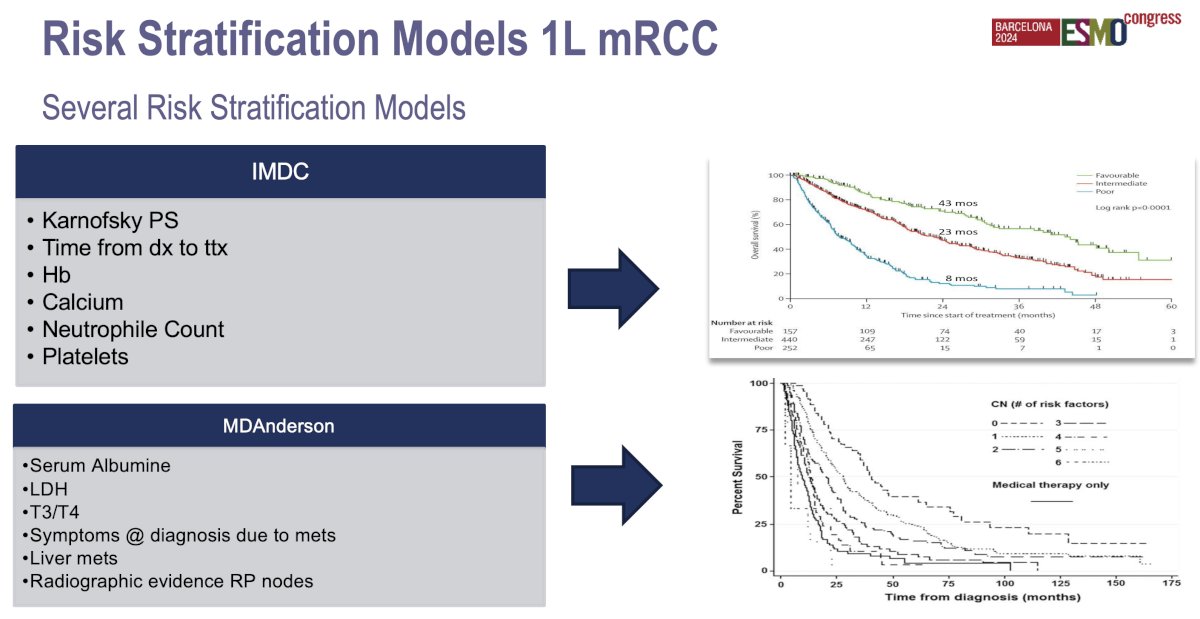
Multiple risk stratification criteria and systems have been developed, but very few of these were specifically designed for optimizing patient selection for cytoreductive nephrectomy. The two most commonly used classification systems for prognosticating patient outcomes are the MSKCC and IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) risk stratification systems, which were designed to stratify patients by expected overall survival following systemic therapy treatment.
The IMDC model is similar to the MSKCC scoring scheme, but lactate dehydrogenase was removed, and two risk factors (elevated neutrophil count and thrombocytosis) were added. Similarly, patients are also stratified into favorable- (0 risk factors), intermediate- (1–2), and poor-risk (≥3) categories. These risk classification schemes provide prognostic information for patients with metastatic RCC. Dr. Mir noted that these systems are often relied upon when determining patient eligibility for cytoreductive nephrectomy; however, neither system was developed with the intention of identifying appropriate candidates for cytoreductive nephrectomy.
A risk classification system to predict outcomes following cytoreductive nephrectomy was developed by investigators from the MD Anderson Cancer Center. Their study was specifically designed to evaluate preoperative clinical variables that could be used by clinicians to select ideal candidates for cytoreductive nephrectomy. In a cohort of 566 patients with metastatic RCC who underwent cytoreductive nephrectomy, seven pre-operative variables were independently associated with inferior overall survival, compared with patients from a non-surgical cohort.6
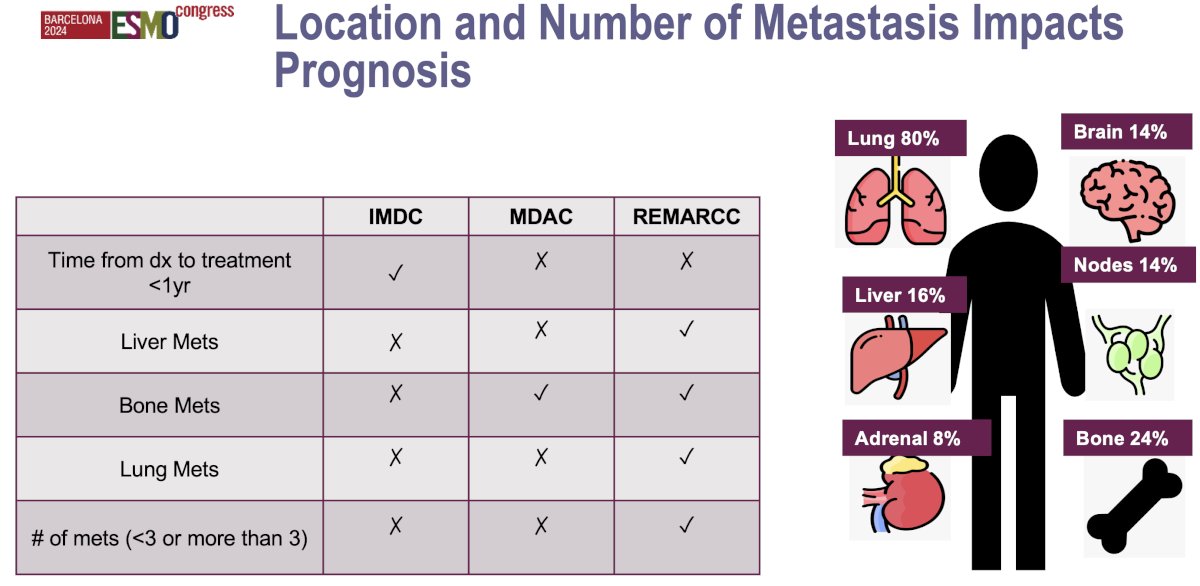
The location and number of metastases both significantly impact prognosis and treatment decision-making. Patients with oligometastases (commonly defined as ≤3–5 metastatic sites), generally have a better prognosis compared to those with widespread, diffuse metastases. Oligometastatic patients may benefit from more aggressive interventions. The location of metastases also plays a crucial role. Metastases in more favorable locations, such as the lungs, are often associated with better outcomes, as these can sometimes be surgically resected or treated with targeted radiotherapy or immunotherapy. In contrast, metastases to the liver or bone are generally associated with a poorer prognosis due to higher metastatic burden and resistance to systemic treatments.
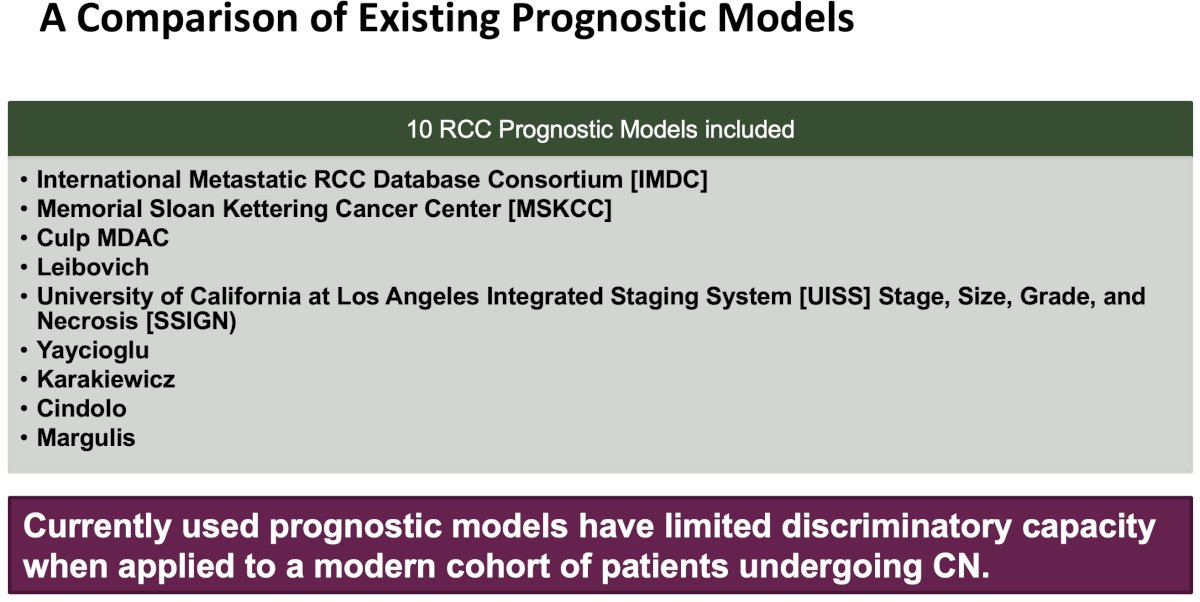
Unfortunately, all risk stratification models to date have shown limited prognostic accuracy when applied to external cohorts. The best prognostic model to date is the Leibovich model, which has a c-index of 0.61. However, the utility of this model in the preoperative cytoreductive nephrectomy setting is limited, considering that pathological variables such as nuclear grade and coagulative necrosis are incorporated into the model. The c-index values for the IMDC and MSKCC models are 0.56 and 0.55, respectively, which approximate the predictive accuracy of a coin flip.7
The two major randomized trials that have recently questioned the benefit of immediate/upfront cytoreductive nephrectomy in metastatic RCC patients are SURTIME and CARMENA. In the non-inferiority CARMENA trial, 411 patients were randomized to sunitinib or cytoreductive nephrectomy followed by sunitinib. This trial demonstrated that upfront sunitinib was associated with superior overall survival outcomes (18.4 versus 13.9 months). This trial was suspended for enrollment earlier than expected due to slow recruitment and a low number of events at the interim analysis. Notable limitations to the CARMENA trial included:
- Eight years were needed to recruit the study cohort
- Patients were unwilling to undergo randomization
- Crossover between arms was significant: 17% of patients in the sunitinib arm underwent a deferred nephrectomy
- CARMENA patients were generally sicker and had a higher metastatic disease burden – factors known to make patients less likely to benefit from a cytoreductive nephrectomy
The SURTIME trial randomized metastatic RCC patients to immediate cytoreductive nephrectomy followed by sunitinib therapy versus treatment with 3 cycles of sunitinib followed by cytoreductive nephrectomy in the absence of progression followed by sunitinib therapy. Progression-free survival was the primary endpoint, which required a sample size of 458 patients. Because of poor accrual, the independent data monitoring committee endorsed reporting the intention-to-treat 28-week progression-free rate instead. The study closed after 5.7 years with 99 patients enrolled. The intention-to-treat overall survival hazard ratio favored the deferred cytoreductive nephrectomy arm (HR: 0.57, p=0.03), with median overall survivals of 32.4 and 15 months, respectively. It was clear, however, that if patients progressed within 3 months, irrespective of treatment approach, they all had poor prognoses.
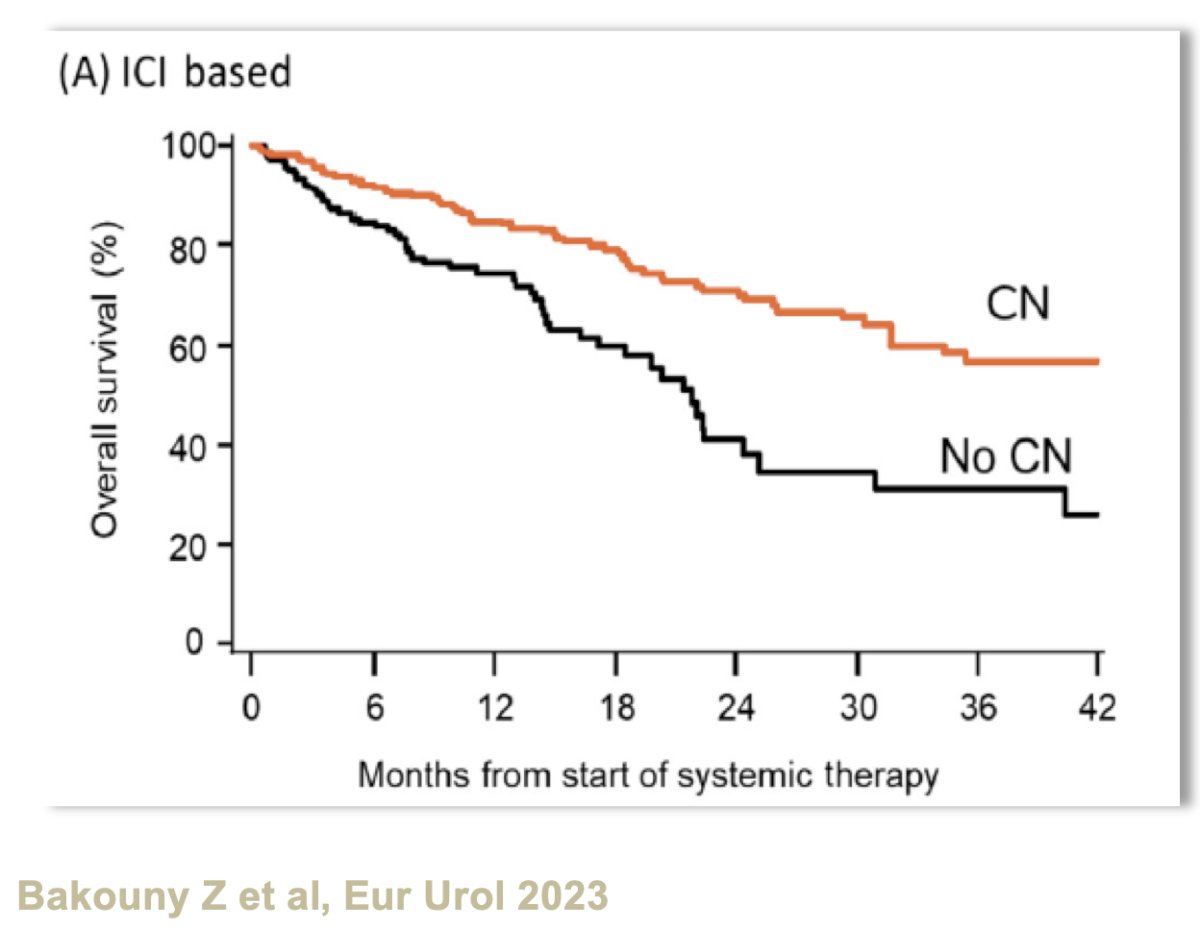
Recently, an observational study from the IMDC database evaluated the benefits of upfront cytoreductive nephrectomy for metastatic RCC patients treated with immune checkpoint inhibitors or targeted therapy. Cytoreductive nephrectomy was associated with significant overall survival benefits in both the immune checkpoint inhibitor- (HR: 0.61, p=0.013) and targeted therapy-treated groups (HR: 0.72, p<0.001).8 The median overall survivals were 25 versus 13 months in patients receiving 1st line targeted therapy and 54 versus 22 months for those receiving 1st line immune checkpoint inhibitors (both favoring cytoreductive nephrectomy). The survival advantage was most significant for patients with favorable prognostic factors, such as limited metastatic burden, good performance status, and absence of IMDC poor-risk features. In contrast, for patients with poor-risk features or a high metastatic burden, upfront cytoreductive nephrectomy was associated with worse survival outcomes, suggesting that these patients benefit more from systemic therapies as the initial treatment strategy. Overall, this study reinforces the contemporary trend towards a more selective approach to the selection of patients for cytoreductive nephrectomy, whereby only well-selected patients with favorable clinical profiles might benefit from surgery, whereas most other patients should receive upfront systemic therapy.
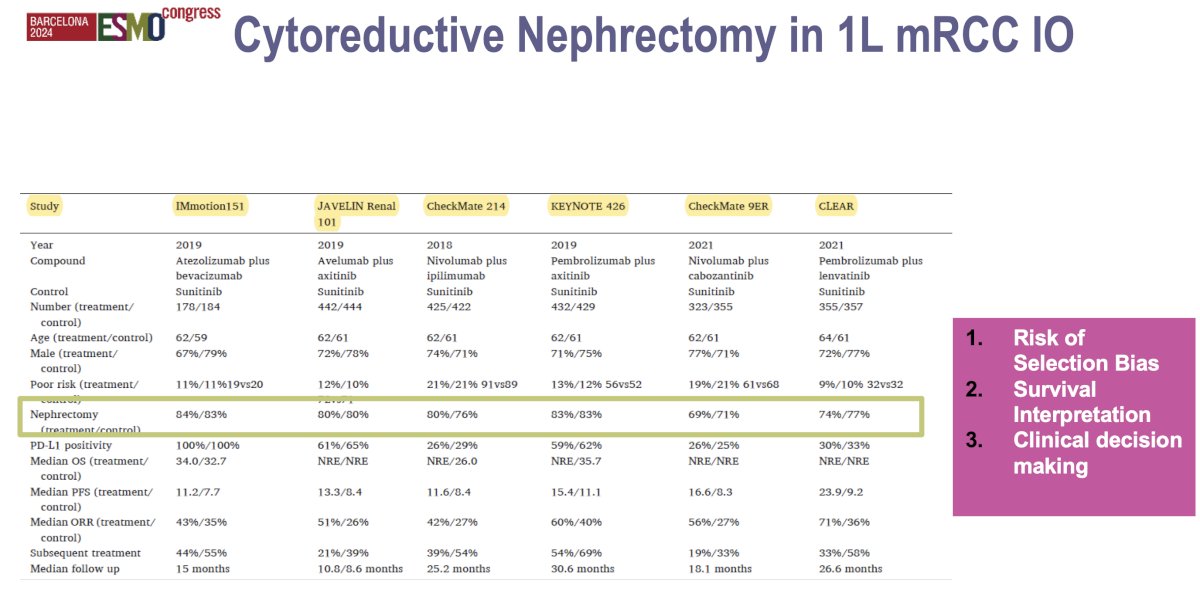
One key takeaway from the trials of 1st line immune checkpoint inhibitor combinations is that the majority of enrolled patients had undergone a prior radical nephrectomy (≥70% across the trials). This has important consequences/limitations when attempting to apply the results of these trials to patients with de novo metastatic disease being considered for cytoreductive nephrectomy:
- Patient selection bias: These trials may have enrolled a generally healthier population with better performance status and lower metastatic burden, as patients who undergo nephrectomy tend to be fitter. This could lead to overestimation of the efficacy of immunotherapy in the general metastatic RCC population, as results may not fully represent patients with more advanced disease or those who were not candidates for nephrectomy.
- Survival interpretation: Patients who had already undergone a nephrectomy prior to trial enrolment might have had an inherently better prognosis due to tumor debulking and better baseline status. This may skew the overall survival and progression-free survival data, making it harder to determine how much of the survival benefit was due to immunotherapy versus the prior nephrectomy.
- Applicability of results: The outcomes of these trials may not fully apply to patients with metastatic disease who have not undergone nephrectomy, especially those with poor-risk features or extensive metastatic burden. It raises questions about whether the same immunotherapy benefits would apply in the absence of tumor debulking from nephrectomy.
- Clinical decision making: The trials provide limited data on how patients who have not had a nephrectomy respond to first-line immunotherapy, which complicates treatment decisions for these individuals. This uncertainty reinforces the need for more data in ‘non-nephrectomized’ metastatic RCC patients to better understand the role of immunotherapy in this group
What about deferred or consolidative cytoreductive nephrectomy? The rationale for this approach, as opposed to upfront cytoreductive nephrectomy, includes the following:
- Some patients might not need a cytoreductive nephrectomy if a complete response is achieved
- Upfront systemic therapy is an opportunity to identify patients with poor risk that could potentially benefit from cytoreductive nephrectomy
- Timely systemic therapy is an important management component that has demonstrated improved efficacy outcomes
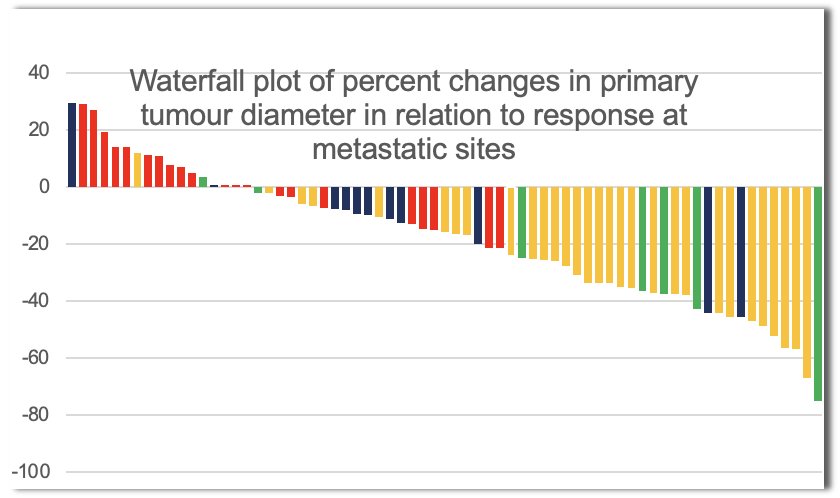
Can we predict a pT0 response in these patients initially receiving systemic therapy? This would obviate the need for unnecessary surgical extirpation. While complete responses to immune checkpoint inhibitors are observed in metastatic sites, achieving a pT0 status in the primary tumor is rare and remains difficult to predict. In the CheckMate 214 trial of 1st line ipilimumab + nivolumab, no patient with an evaluable primary renal tumor achieved a complete response (≥30% reduction in 35% of patients).9 Similarly, there were no complete responses in 67 patients with intermediate or poor prognosis disease receiving the triplet combination of nivolumab + ipilimumab + cabozantinib.10
Several factors may help predict a pT0 response:
- Baseline tumor characteristics: Smaller primary tumors with lower stage and grade at diagnosis may be more likely to fully respond to immunotherapy. Tumors with less aggressive histologic features might exhibit better immune responsiveness.
- Tumor microenvironment: The presence of an inflamed tumor microenvironment (with high levels of immune cells like T-cells) may predict a more robust response to immunotherapy. Expression of biomarkers like PD-L1 could also suggest a better likelihood of achieving pT0.
- Systemic response: A strong systemic response to immunotherapy, with significant shrinkage or control of metastatic disease, could correlate with a higher chance of achieving complete pathological regression in the primary tumor.
- Molecular signatures: Ongoing research is investigating genetic and molecular signatures that may predict responses. Some studies suggest that tumors with high mutational burden or specific genomic alterations may be more sensitive to immunotherapy.
A 2022 study by Meerveld-Eggink et al evaluated the primary tumor response in patients with metastatic RCC treated with ipilimumab plus nivolumab. This study attempted to evaluate how the primary renal tumor responds to systemic immunotherapy, as most studies have focused on metastatic sites, not the primary tumor. The study results demonstrated that the primary tumors did respond to therapy, with significant reductions in size observed in a portion of patients. However, the response rate in the primary tumor was generally lower compared to the response rates seen at metastatic sites. There was significant variability in the primary tumor’s response, which correlated with factors such as baseline tumor characteristics and immune environment. These findings suggest that while immunotherapy is effective at controlling metastatic disease, the primary tumor may be less responsive, raising important considerations for the role of cytoreductive nephrectomy.11
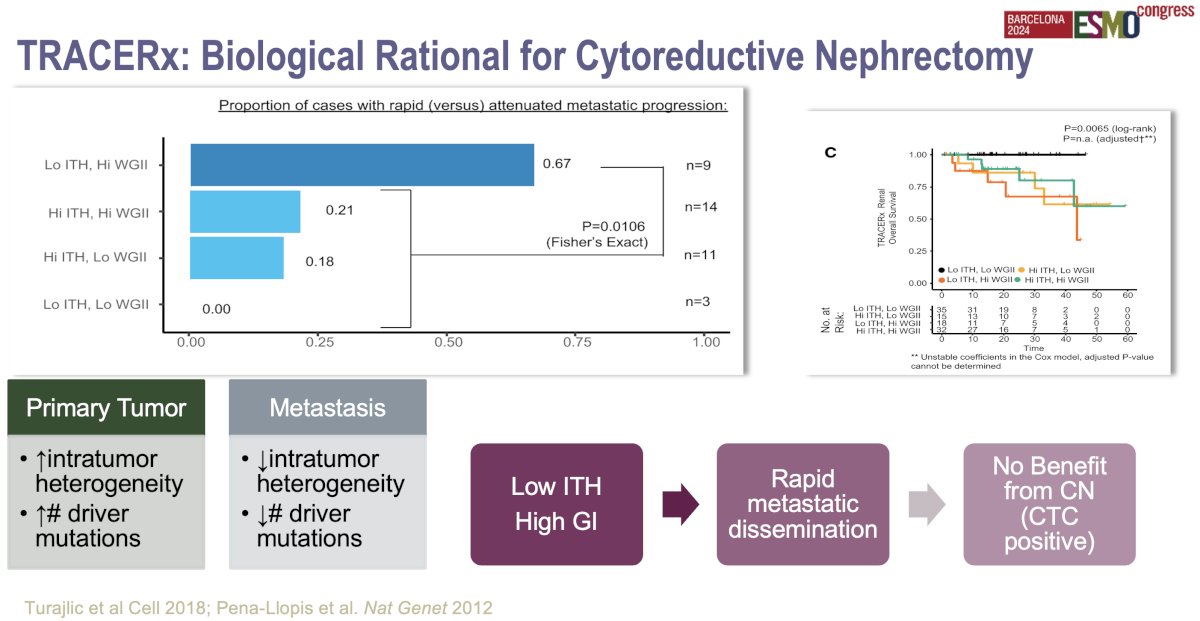
The biological rationale for cytoreductive nephrectomy in metastatic RCC has been further clarified by findings from the TRACERx Renal study, which investigated tumor evolution and heterogeneity.12 Key insights include:
- Tumor heterogeneity: TRACERx demonstrated that kidney cancer is highly heterogeneous, meaning that different parts of the primary tumor and its metastases can have distinct genetic mutations. By removing the primary tumor via cytoreductive nephrectomy, there may be a reduction in the overall tumor burden and the genetic complexity of the disease, potentially decreasing the emergence of resistant clones that could drive disease progression.
- Metastatic seeding: The study found that primary tumors can continue to seed metastases over time. By performing cytoreductive nephrectomy, this source of new metastatic spread is eliminated, possibly slowing disease progression. This is particularly relevant in patients with a low metastatic burden, where metastases might still be primarily dependent on the primary tumor for growth signals or genetic material.
- Immune microenvironment: Cytoreductive nephrectomy may also improve the immune response. The primary tumor can suppress immune function through various mechanisms, including the release of immunosuppressive factors. By removing the tumor, the immune system may be better able to recognize and attack remaining metastatic cells, especially when combined with immunotherapy.
These findings support cytoreductive nephrectomy in select patients, potentially improving systemic therapy effectiveness and long-term outcomes.
Dr. Mir noted that ongoing questions with regard to deferred cytoreductive nephrectomy include the following:
- How long do we give systemic therapy prior to cytoreductive nephrectomy?
- What is the degree of response (downstaging) that should be achieved prior to cytoreductive nephrectomy? Is a partial response good enough?
- What patients can discontinue systemic therapy following cytoreductive nephrectomy?
What we need is improved patient selection criteria using risk stratification techniques that incorporate biologic signals (i.e., ctDNA). For the time being, treatment decision making in this setting needs to be guided by a multidisciplinary team-guided approach.
Ongoing trials in this space include:
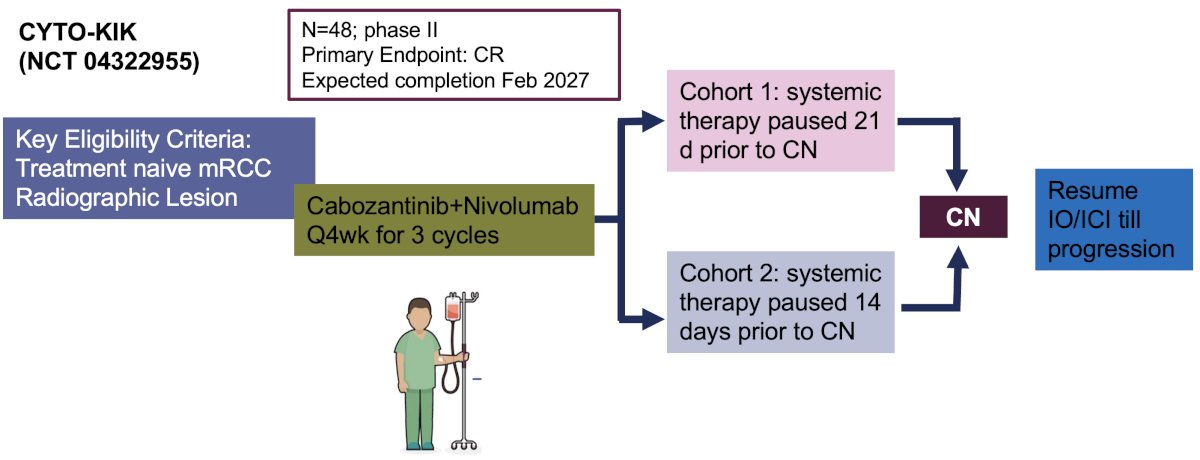
- CYTO-KIK (NCT04322955): Phase II trial of 48 patients that will receive 3 cycles of cabozantinib + nivolumab and then undergo cytoreductive nephrectomy either 21 days (Cohort 1) or 14 days (Cohort 2) later, after which systemic therapy will be resumed till disease progression
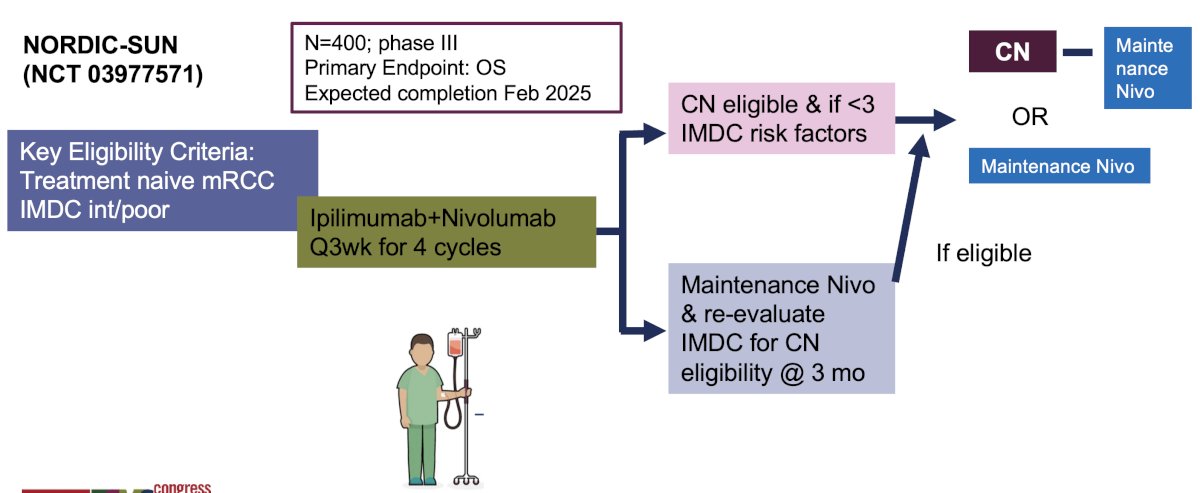
- NORDIC-SUN (NCT03977571): Phase III trial of 400 patients receiving 4 cycles of ipilimumab + nivolumab followed by cytoreductive nephrectomy if <3 IMDC risk factors versus maintenance nivolumab with re-evaluation for cytoreductive nephrectomy eligibility at 3 months.
- PROBE (NCT4510597): Phase III trial of 364 patients who will receive 12 weeks of immune checkpoint inhibitor combinations with cytoreductive nephrectomy performed in eligible patients who achieve a partial response or have stable disease.
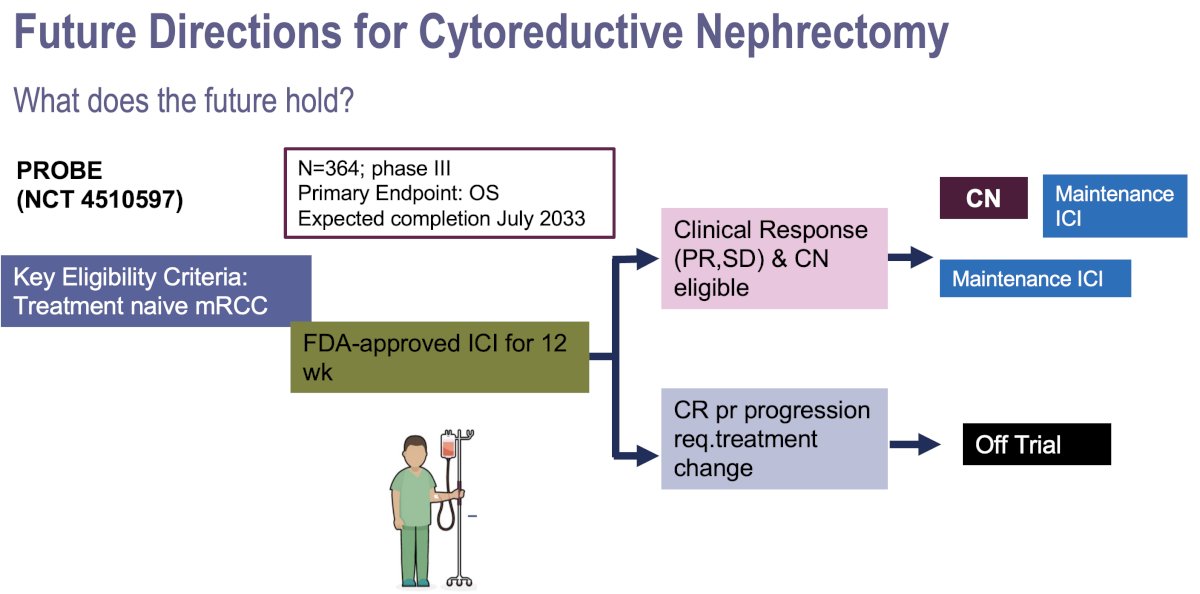
Dr. Mir concluded her presentation with the following evidence-based management algorithm for cytoreductive nephrectomy in patients with de no metastatic RCC:

Presented by: Maria Carmen Mir, MD, PhD, FEBU, Department of Urology, Fundacion Instituto Valenciano Oncologia, Valencia, Spain
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. N Engl J Med. 2001; 345:1655-9.
- Mickisch GHJ, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001; 358(9286):966-70.
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med. 2018; 379(5):417-27.
- Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019; 5(2):164-70.
- Bhindi B, Abel EJ, Albiges L, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol. 2019; 75(1):111-28.
- Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010; 116(14):3378-88.
- Westerman ME, Shapiro DD, Tannir NM, et al. Survival following cytoreductive nephrectomy: a comparison of existing prognostic models. BJU Int. 2020; 126(6):745-53.
- Bakouny Z, El Zarif T, Dudani S, et al. Upfront Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors or Targeted Therapy: An Observational Study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2023; 83(2):145-51.
- Albiges L, Tannir NM, Burotto M, et al. First-line Nivolumab plus Ipilimumab Versus Sunitinib in Patients Without Nephrectomy and With an Evaluable Primary Renal Tumor in the CheckMate 214 Trial. Eur Urol. 2022; 81(3):266-71.
- Iacovelli R, Ciccarese C, Maruzzo M, et al. Primary Tumor Shrinkage and the Effect on Metastatic Disease and Outcomes in Patients With Advanced Kidney Cancer With Intermediate or Poor Prognosis Treated With Nivolumab Plus Ipilimumab or Cabozantinib. Clin Genitourin Cancer. 2022; 20(5):498.e1-9.
- Meerveld-Eggink A, Graadland N, Wilgenhof S, et al. Primary Renal TumourResponseinPatients Treated with Nivolumab and Ipilimumab for Metastatic Renal Cell Carcinoma: Real-world Data Assessment. Eur Urol Open Sci. 2022; 35:54-8.
- Turajlic S, Xu H, Litchfield K, et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell. 2018; 173(3):595-610.


