(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Updates in the management of advanced renal cell carcinoma. Dr. Teresa Alonso Gordoa discussed systemic treatment options for the management of advanced renal cell carcinoma.
Dr. Alonso Gordoa began her presentation by discussing the dramatic improvement in survival for metastatic renal cell carcinoma (mRCC) over the past 25 years. In 1999, the median overall survival (OS) reported by Motzer at the Memorial Sloan Kettering Cancer Center (MSKCC) was 10 months for the overall population, 19.9 months for patients with favorable prognosis, 10.3 months for those with intermediate prognostic group, and 3.9 months for those in the poor prognostic group.1 Today, the median OS (CheckMate 214) has increased up to five times to 52.7 months for the overall population, up to four times to 77.9 months for the favorable prognostic group, five times for the intermediate prognosis group, and up to nine times to 48.1 months for the poor prognosis group.2 These improvements are attributed to immunotherapy (IO)-based combination therapies, treatment sequencing, novel agents, improved local therapies, and increased safety in the management of mRCC.
First line treatment
The first-line treatment of metastatic renal cell carcinoma (mRCC) has changed significantly over the last decade and now includes three therapeutic targets and their combinations. The first target is Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a protein receptor that functions as an immune checkpoint and downregulates immune responses. CTLA-4 is constitutively expressed in regulatory T cells but is only upregulated in conventional T cells after activation. The second target is Programmed Death 1 (PD-1), which plays a vital role in inhibiting immune responses and promoting self-tolerance by modulating the activity of T cells. The third target is the vascular endothelial growth factor receptor (VEGFR), targeted by tyrosine kinase inhibitors. Recently, five major studies have shaped the treatment landscape of mRCC.
These studies are the CheckMate2142 which evaluated the combination of IO/IO (nivolumab. And ipilimumab) a PD-1 inhibitor and a CTLA4-inhibitor. The Keynote 426,3 CLEAR4 and Checkmate 9ER,5 these three studies evaluated the combination between IO (anti-PD1) with a VEGFR-TKI. Lastly, the COSMIC 313 trial,6 evaluated triplet therapy which involves the combination of IO+IO+ VEGFR-TKI (nivolumab + ipilimumab + cabozantinib).
Dr. Alonso Gordoa then discussed the CheckMate 214 trial, a phase 3 study comparing nivolumab plus ipilimumab with sunitinib for previously untreated clear-cell advanced renal-cell carcinoma (ccRCC) with intermediate or poor prognostic risk. Patients were randomly assigned in a 1:1 ratio to receive either nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) intravenously every 3 weeks for four doses, followed by nivolumab (3 mg/kg) every 2 weeks, or sunitinib (50 mg) orally once daily for 4 weeks (6-week cycle). At a minimum median follow-up of 99 months, the overall response rate (ORR) was 42.4% for nivolumab plus ipilimumab versus 27.5% for sunitinib, with 11.8% in the experimental arm achieving a complete response compared to 2.6% in the sunitinib arm. Impressively, the median duration of response (DOR) was 82.8 months vs. 19.8 months.2

Moreover, there was a significant benefit in the group treated with nivolumab plus ipilimumab in terms of overall survival (OS) and progression-free survival (PFS). The median overall survival was 46.7 months in the nivolumab plus ipilimumab arm versus 26 months in the sunitinib arm. The OS was significantly better in all analyses, starting at 25 months and continuing through the latest analysis at 99 months.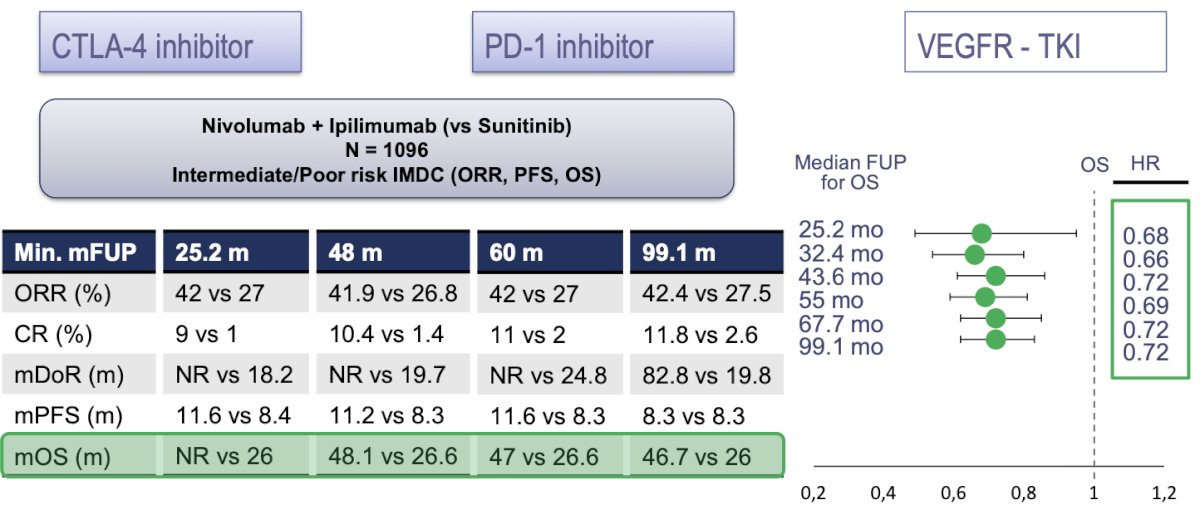
Interestingly, when analyzing only patients in the favorable risk group, there was no significant benefit in terms of overall survival (OS) (HR 0.82, 95% CI 0.60-1.13) or progression-free survival (PFS) (HR 1.76, 95% CI 1.25-2.48). However, the duration of response was almost double for the nivolumab plus ipilimumab arm (median 61.5 months vs. 33.2 months), although 53.6% had progressive disease in the nivolumab plus ipilimumab arm compared to 35.5% in the sunitinib arm.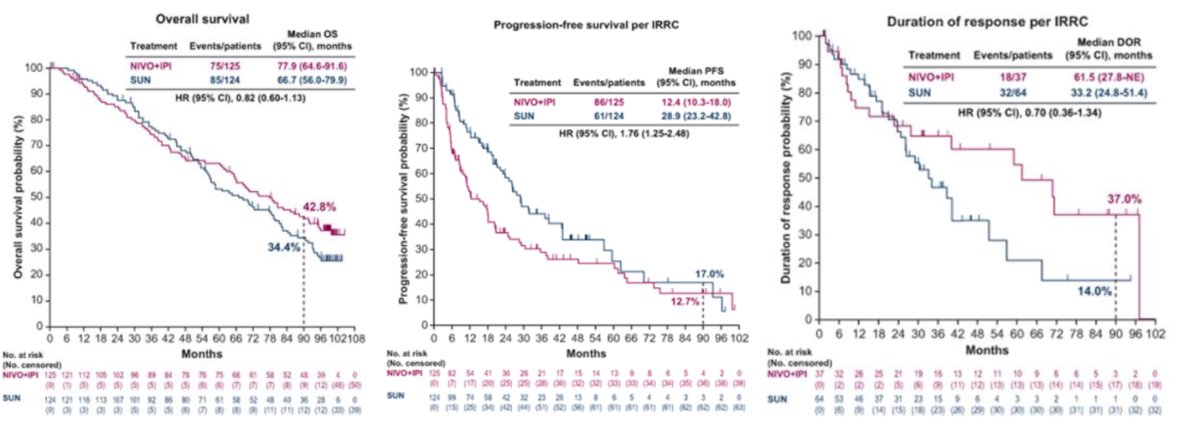
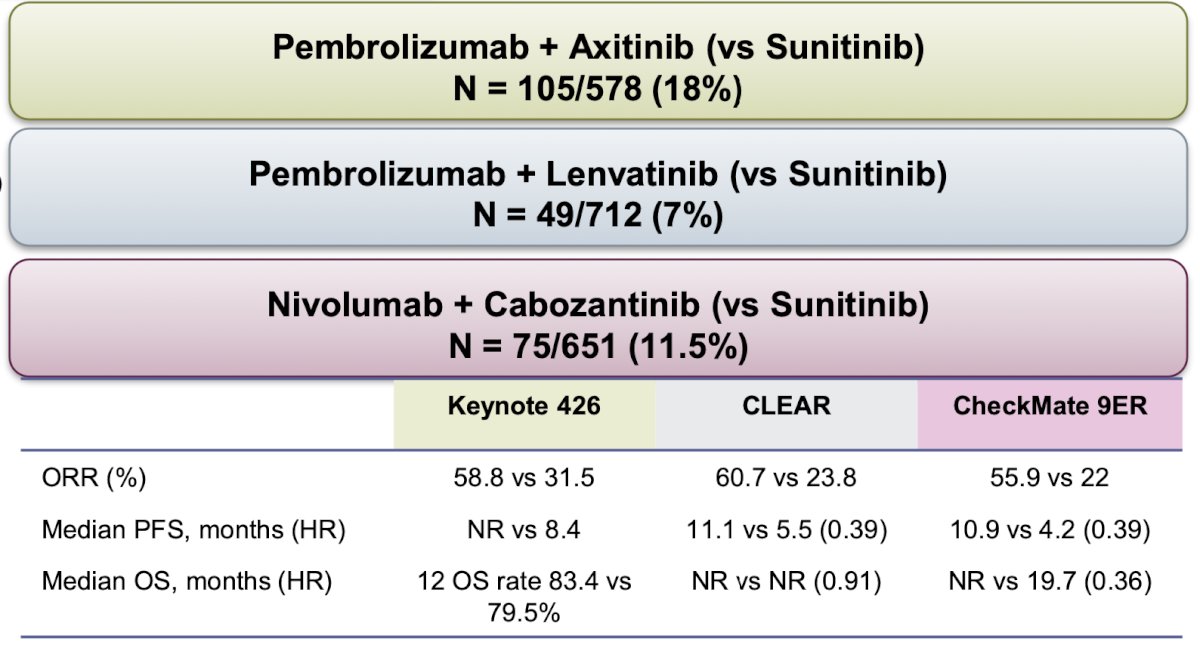
Three trials compared the combination of a PD-1 inhibitor and a VEGFR-TKI for advanced ccRCC. The first was the Keynote 426, a phase 3 trial that randomly assigned 861 patients with previously untreated advanced ccRCC to receive either pembrolizumab (200 mg intravenously once every 3 weeks) plus axitinib (5 mg orally twice daily) (432 patients) or sunitinib (50 mg orally once daily).3 The second was the CLEAR trial, an open-label, phase 3, randomized trial that enrolled patients with advanced ccRCC in a 1:1:1 ratio to receive lenvatinib (20 mg per day orally) plus pembrolizumab (200 mg intravenously every 21 days), lenvatinib (18 mg per day orally) plus everolimus (5 mg per day orally), or sunitinib (50 mg per day orally, 4 weeks on and 2 weeks off).4 The third was the CheckMate 9ER trial, a phase 3, randomized, open-label trial that assigned patients with advanced ccRCC to receive either nivolumab (240 mg every 2 weeks) plus cabozantinib (40 mg once daily) or sunitinib (50 mg once daily for 4 weeks of each 6-week cycle).5
There were some differences in terms of IMDC risk group between these trials (more IMD favorable group in Keynote 426 and CLEAR), more patients with poor prognostic group (19%), and less patients with prior nephrectomy (69%) in CheckMate 9ER.
The overall response rate (ORR) across the three trials surpassed that of sunitinib, as outlined in the table and figure below. Impressively, at a minimum median follow-up of 43 months, 10% of patients in Keynote 426, 18% in CLEAR, and 13% in CheckMate 9ER achieved a complete response (CR).
The median overall survival (OS) at the longest follow-up in each trial was as follows: 46 months for pembrolizumab plus axitinib versus 40 months for sunitinib in Keynote 426; 54 months for lenvatinib plus pembrolizumab versus 54 months for sunitinib in CLEAR; and 50 months for cabozantinib plus nivolumab versus 36 months for sunitinib in CheckMate 9ER.
The combination of immunotherapy (IO) with IO did not appear to be associated with a higher rate of treatment-related adverse events (TRAEs) or Grade 3-5 TRAEs. Among the combinations, lenvatinib plus pembrolizumab, followed by pembrolizumab plus axitinib, had the highest rates of TRAEs and Grade 3 TRAEs.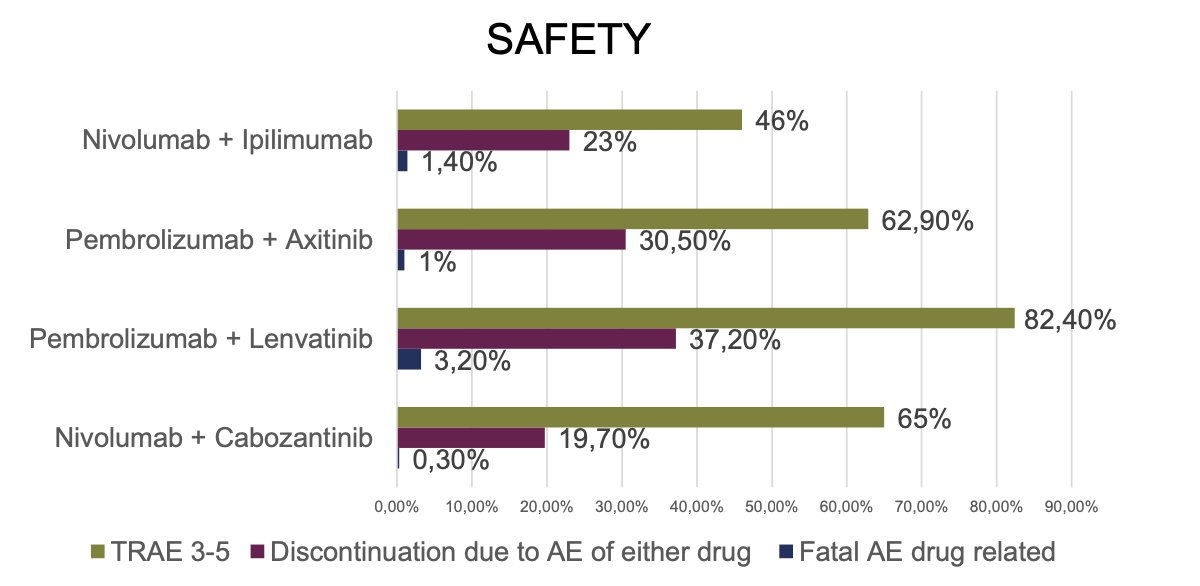
In terms of health-related quality of life (HRQoL), the CheckMate 214 and CheckMate 9ER trials showed that the IO/IO and IO/TKI combinations, respectively, were associated with a significant benefit over sunitinib. However, this benefit was not observed in the CLEAR or Keynote 426 studies. This discrepancy raises the question of whether we can modify treatment intensity in the first-line setting of metastatic renal cell carcinoma (mRCC).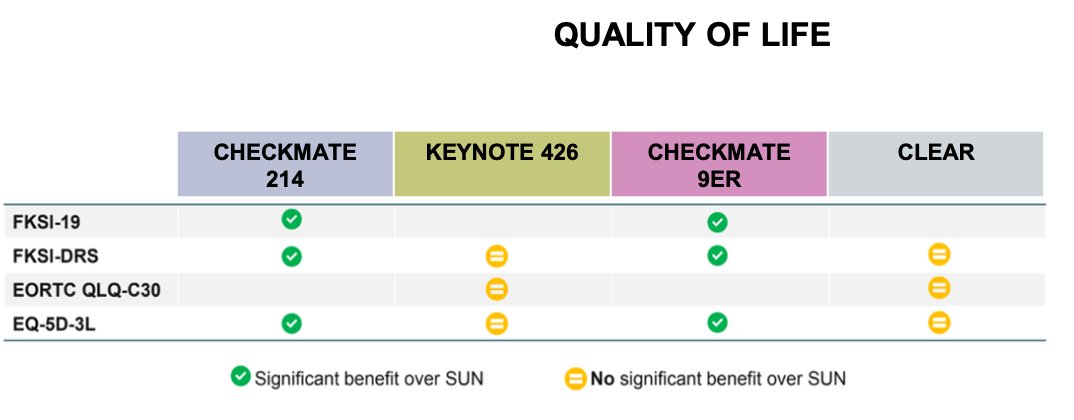
De-escalation strategies have been proposed and are a topic of ongoing research in metastatic renal cell carcinoma (mRCC). The table below outlines the de-escalation strategies employed in the four trials previously mentioned. Most of these strategies include protocol amendments that allow for the discontinuation of one or the two drugs or a maximum number of cycles (e.g., Pembrolizumab).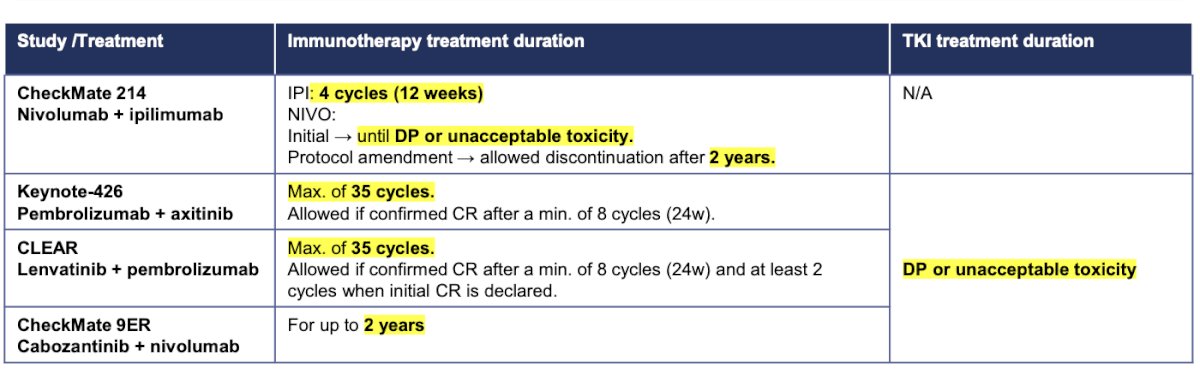
The STAR an open-label, non-inferiority, randomized, controlled, phase 2/3 trial in patients with advanced or inoperable ccRCC randomly assigned participants (1:1) to either a conventional continuation strategy or a drug-free interval strategy. All patients initially received standard dosing schedules of oral sunitinib (50 mg per day) or oral pazopanib (800 mg per day) for 24 weeks before moving into their randomly allocated group. The results indicated no clinically meaningful reduction in life expectancy between the drug-free interval strategy and the conventional continuation strategy groups (HR for OS 0.97 [95% CI 0.83 to 1.12]). This suggests that treatment interruption might be a feasible option, potentially offering lifestyle benefits for patients undergoing TKI therapy.7
In the CheckMate 214 trial, a partitioned survival analysis assessed treatment-free survival (TFS) with and without toxicity as components of a partitioned survival model during the 5-year update. In the intent-to-treat (ITT) population, 18% of patients treated with nivolumab plus ipilimumab (NIVO+IPI) and 5% of those treated with sunitinib were surviving treatment-free without subsequent therapy. For patients with favorable risk, the 60-month mean TFS was 14.4 months for NIVO+IPI versus 5.5 months for sunitinib. Additionally, the average time of treatment during the median overall survival (OS) was 11.1 months for NIVO+IPI compared to 4.4 months for sunitinib.8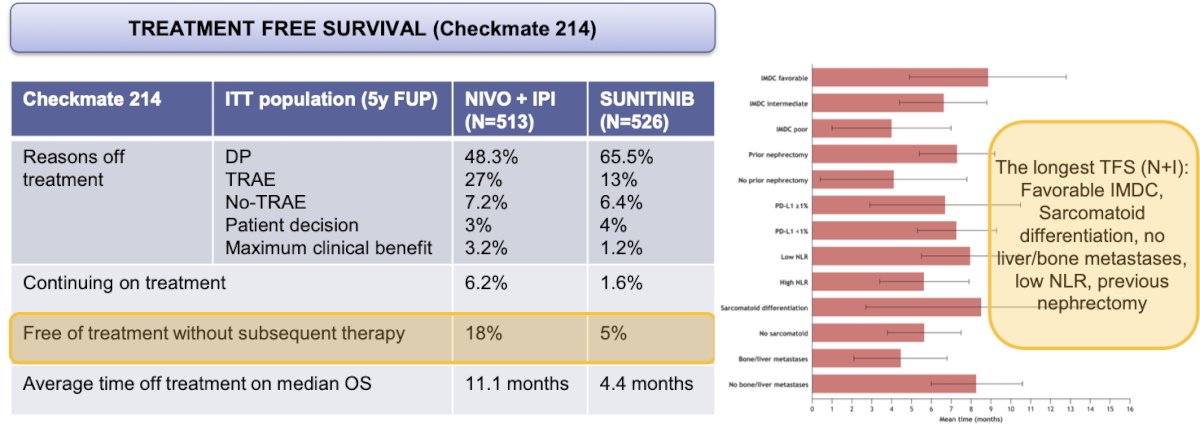
Similar findings were observed in the HCRN GU16-260 Cohort A trial, where eligible patients with treatment-naive clear-cell renal cell carcinoma (ccRCC) received nivolumab until the progression of the disease, unacceptable toxicity, or completion of 96 treatment weeks. Patients with progressive disease before or stable disease at 48 weeks could receive salvage nivolumab/ipilimumab. A partitioned overall survival (OS) and treatment-free survival (TFS) analysis with a median follow-up of 37.7 months showed that most patients who completed protocol therapy (72%) or stopped due to adverse events remained treatment-free for prolonged periods (beyond 12 months).9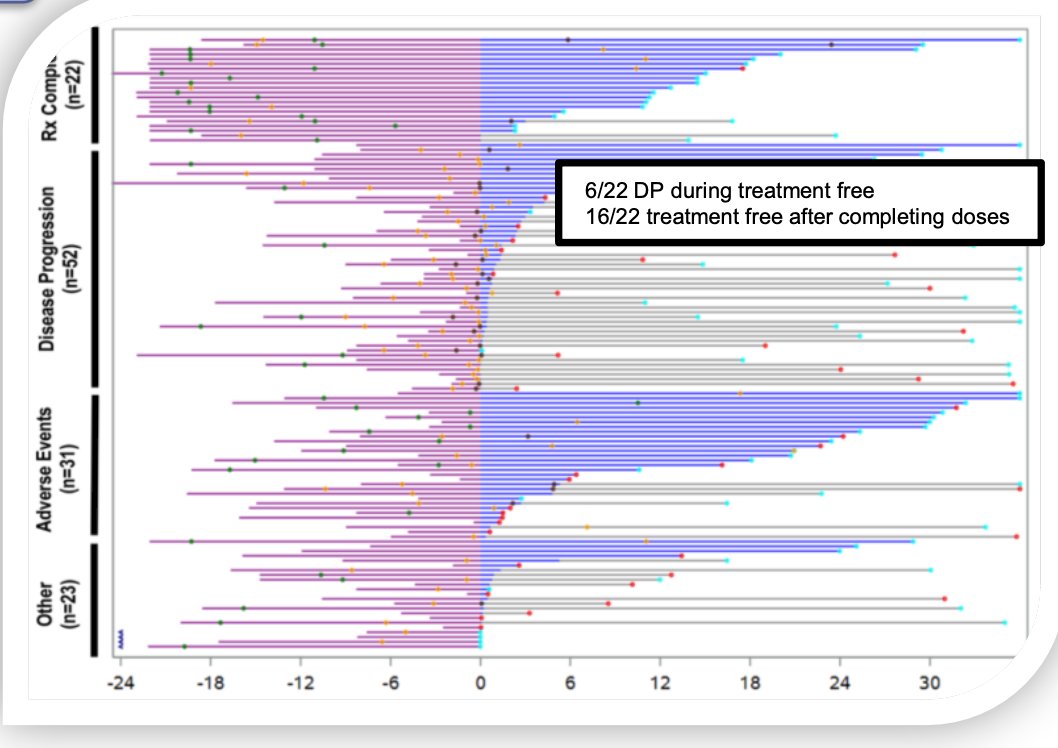
Multiple trials have assessed low-dose and/or increased interval dosing of ipilimumab in combination with PD-1 inhibitors. Early findings suggest that reduced dosing or longer intervals may maintain efficacy while reducing toxicity. However, this approach is not yet standard practice and remains under investigation. Details are shown in the graphic below: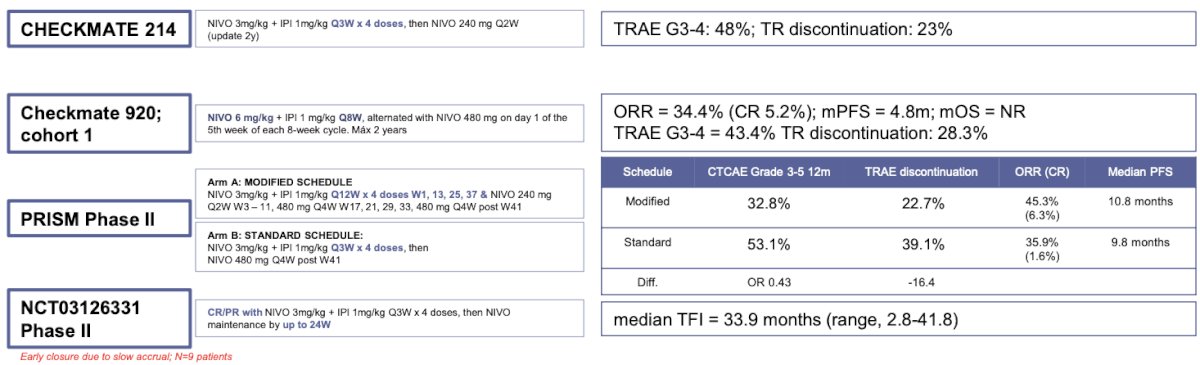
The TIDE-A phase II trial included 75 patients with metastatic clear-cell renal cell carcinoma (ccRCC) who had no primary tumor, no bulky or symptomatic disease, and no liver metastases. Patients received 36 weeks of avelumab 800 mg IV every 2 weeks in combination with avelumab 5 mg orally twice daily. Tumor evaluation was performed at 36 weeks. In patients with a complete or partial response, axitinib was discontinued while avelumab continued at the same dose every 2 weeks until evidence of progressive disease, at which point dual therapy was re-initiated. Patients with stable disease continued the combination until evidence of progression. A total of 26 patients (37%) were able to interrupt the TKI, and 72% had no disease progression at week 8 following TKI discontinuation.10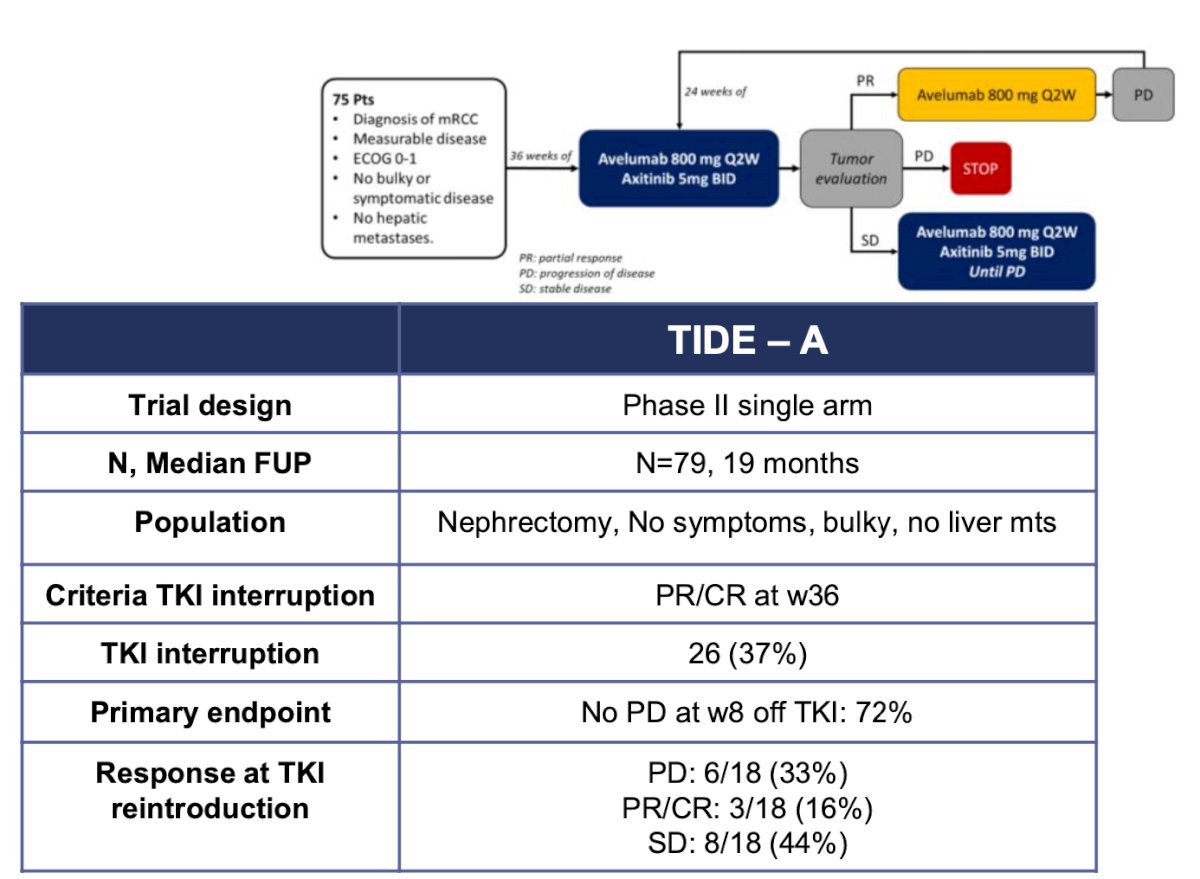
Two studies have evaluated treatment with anti-PD-1 monotherapy: the Keynote 427 and the HCRN GU 16–260 Part A. Details of both trials are summarized in the table below. Notably, the overall response rate (ORR) in Keynote 427 was 36.4%, while in HCRN GU 16–260 Part A it was 34.1%. Interestingly, the ORR was higher in patients with favorable risk (57.1%) compared to those with intermediate/poor risk groups (25%). These findings support the use of single-agent anti-PD-1 therapy in selected patients with favorable risk group disease.9,11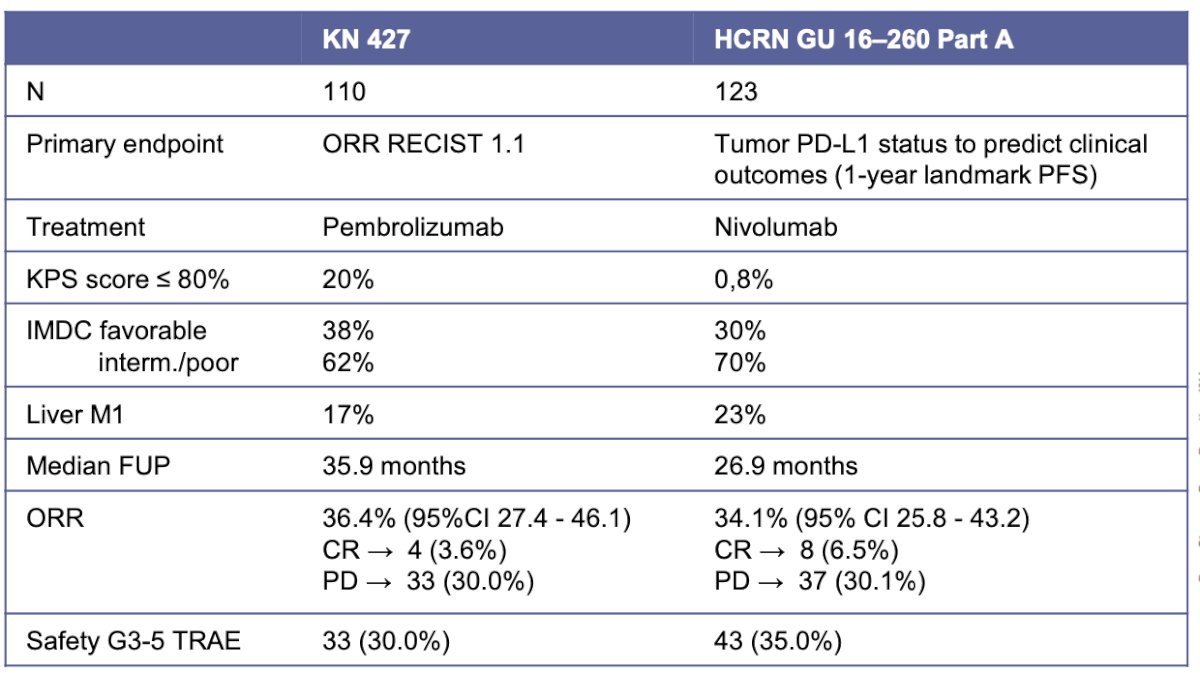
The role of antiangiogenics alone, specifically Belzutifan and Cabozantinib, was evaluated in an open-label, single-arm, phase 2 study involving patients with locally advanced or metastatic ccRCC who had previously received immunotherapy and up to two systemic treatment regimens. Patients were administered Belzutifan 120 mg orally once daily and Cabozantinib 60 mg orally once daily. The study reported a confirmed ORR of 57%, with a median PFS of 30.3 months. Notably, 60% of the patients were categorized in the favorable IMDC risk group.12
Dr. Alonso Gordoa moved on to discuss treatment intensification with the addition of triplets. She reviewed the COSMIC-313 trial, a phase 3, double-blind study involving patients with advanced ccRCC who had not previously received treatment and had intermediate or poor IMDC risk. Patients were randomly assigned to receive either 40 mg of cabozantinib plus nivolumab and ipilimumab (experimental group) or a matched placebo in addition to nivolumab and ipilimumab (control group). The study design is outlined below: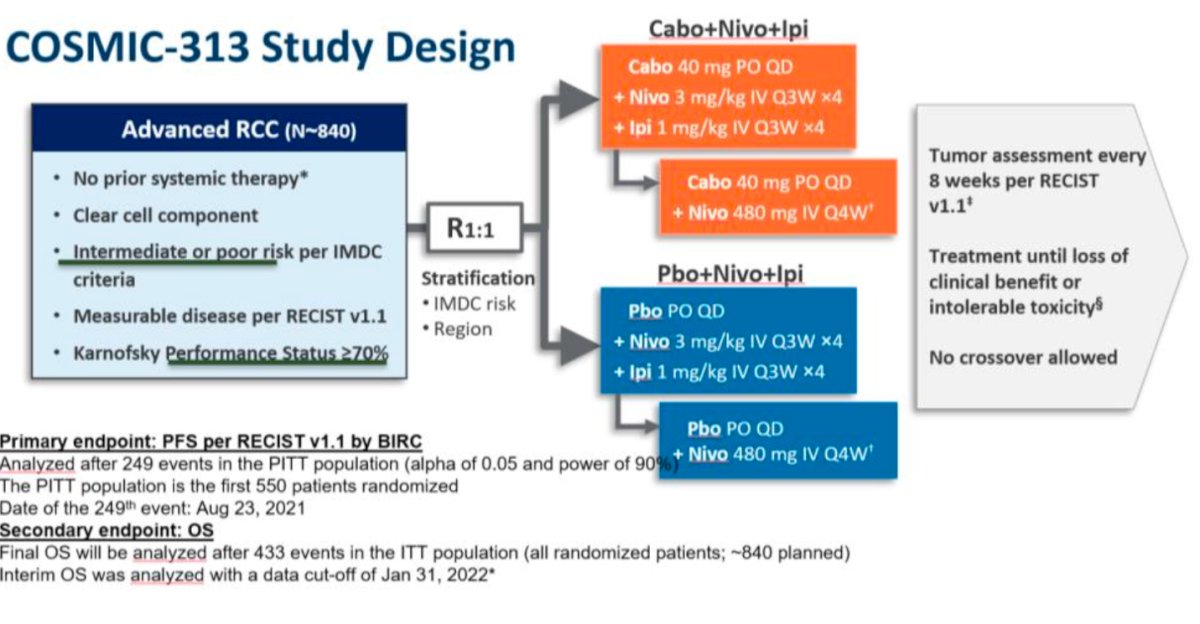
The ORR in the experimental arm was 43% compared to 36% in the control group. The probability of PFS at 12 months was 57% in the experimental group versus 49% in the control group (HR 0.73; 95% CI, 0.57 to 0.94; P = 0.01). However, this benefit came with a higher incidence of TRAEs. Grade 3 or 4 adverse events occurred in 79% of patients in the experimental group, compared to 56% in the control group.6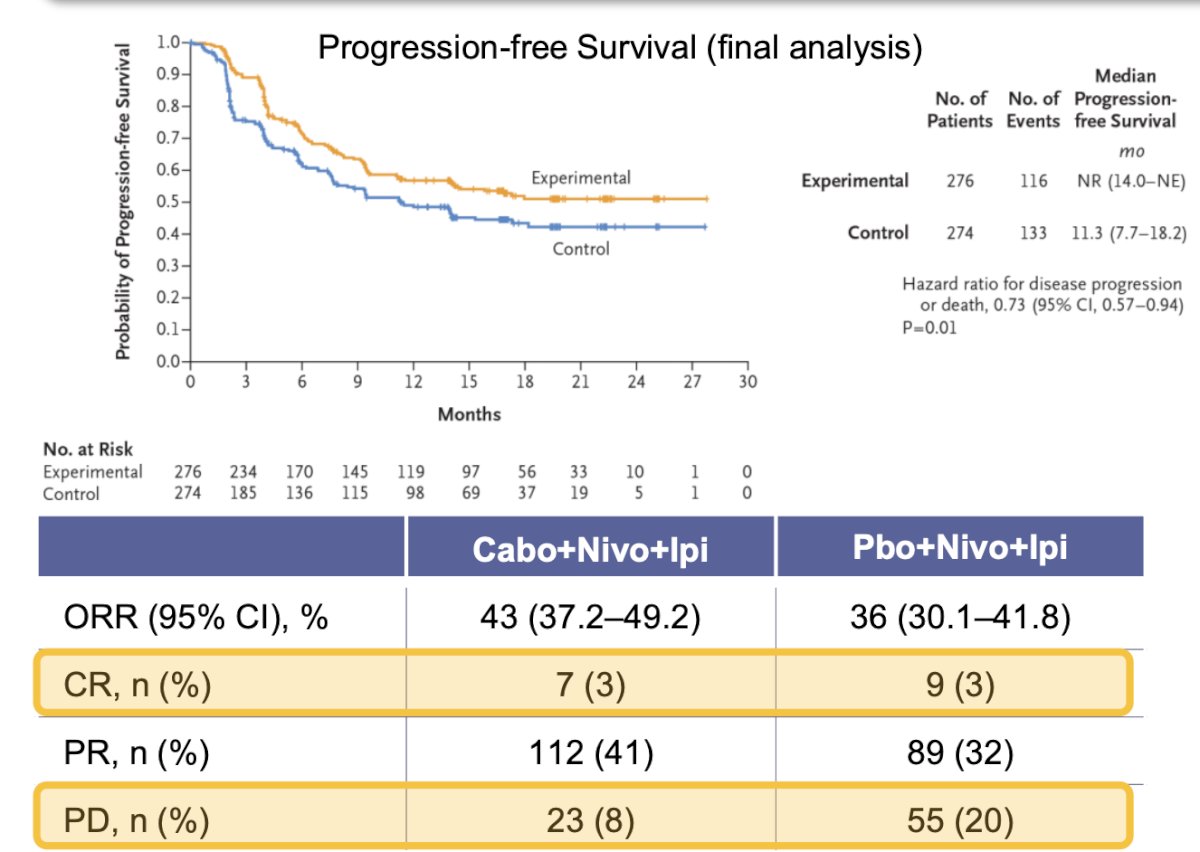
Future strategies in the first-line setting
Future intensification strategies are ongoing such as the NCT04736706. Trial, which is a phase 3 study enrolling patients with advanced ccRCC and randomizing them in three arms:
Arm A: Belzutifan + Pembrolizumab + Lenvatinib
Arm B: Quavonlimab + Pembrolizumab + Lenvatinib
Arm C: Pembrolizumab + Lenvatinib
The BO43936 trial, a phase 3 study, randomizing patients in three arms with arm B examining a triplet (Tobemstomig + tiragolumab + axitinib).
Additionally, adaptive trials like PEDIGREE are underway. In this phase 3 trial, patients will initially receive induction therapy following the CheckMate-214 protocol, which involves ipilimumab 1 mg/kg plus nivolumab 3 mg/kg every three weeks for a total of four cycles. After this induction period, treatment response will be assessed to assign patients to one of three treatment groups. Patients with progressive disease will switch to cabozantinib 60 mg daily. Those with a complete response will proceed with maintenance of nivolumab. For patients with a partial response or stable disease, will be randomly assigned to either maintenance nivolumab alone or nivolumab combined with cabozantinib 40 mg daily.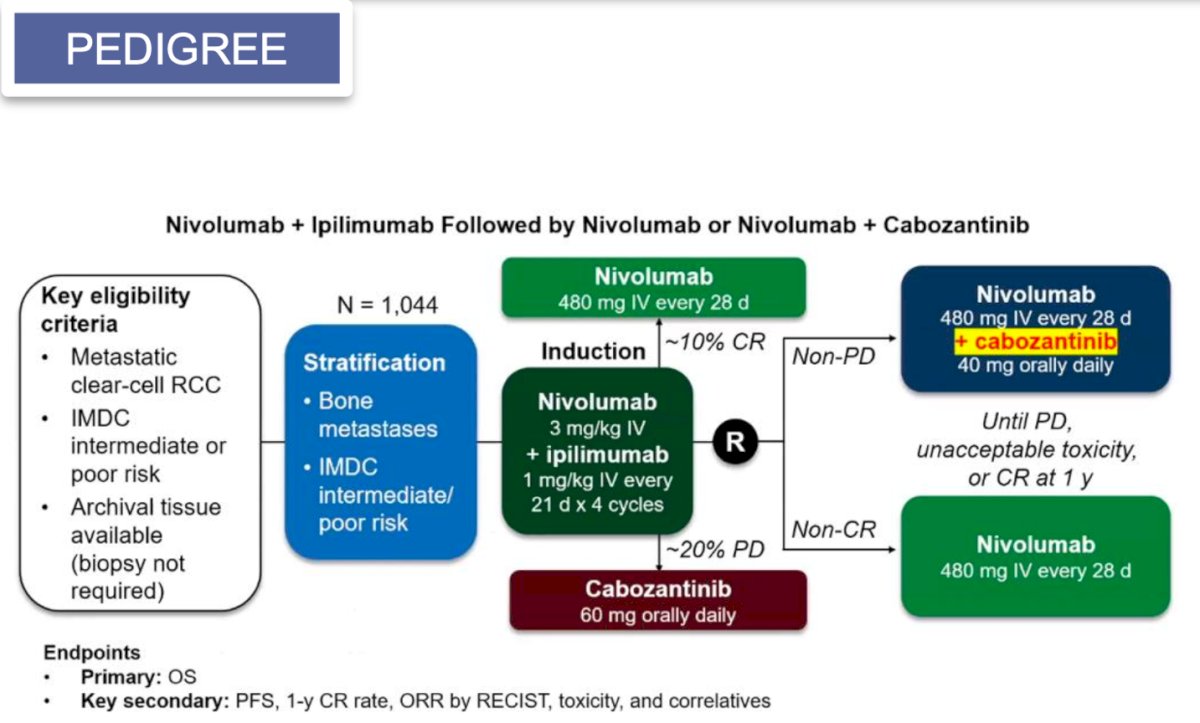
Lastly, the CARE-1 randomized trial, which is the first randomized study platform to optimize biomarkers for treatment in patients with metastatic RCC. Patients will be randomized based on the PD-L1 status as shown in the following trial design: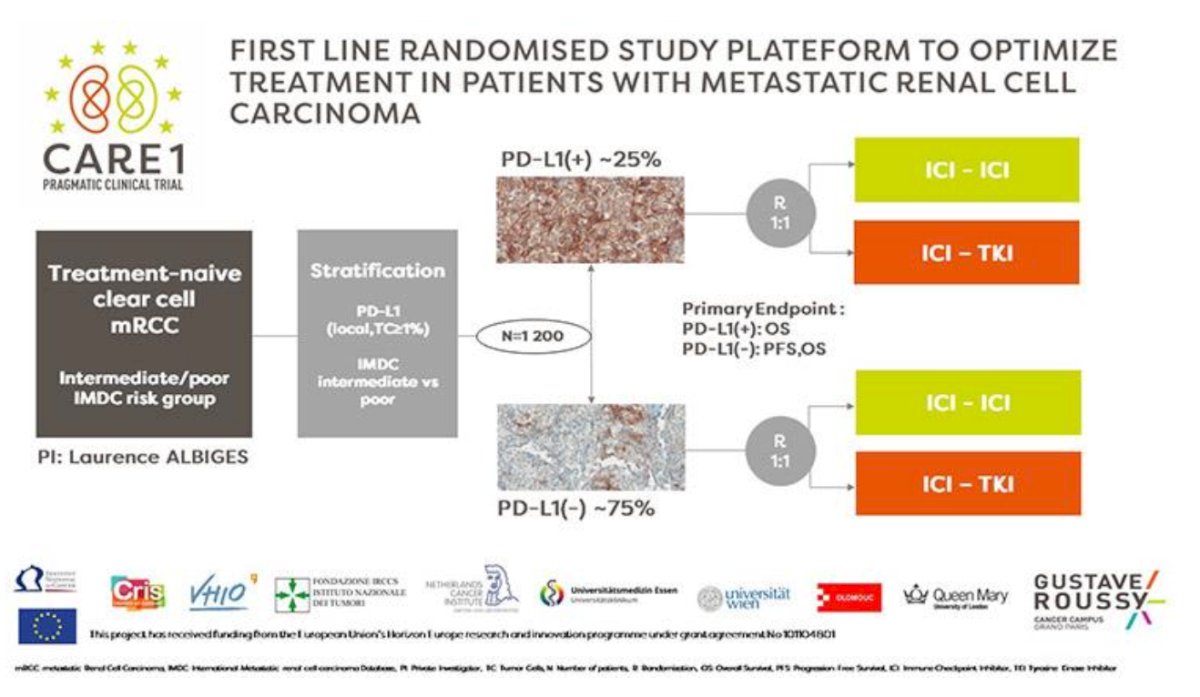
Second and subsequent lines of treatment
The landscape for second and subsequent lines of treatment has become more complex. First, adjuvant pembrolizumab has shown a significant and clinically meaningful improvement in overall survival compared to placebo for patients with clear-cell RCC at high risk of recurrence after surgery.13 In the first-line setting for advanced mRCC, the advent of combinations such as IO+IO, IO+TKI, and IO+IO+TKI has further complicated the decision-making process for second and third-line treatment options.
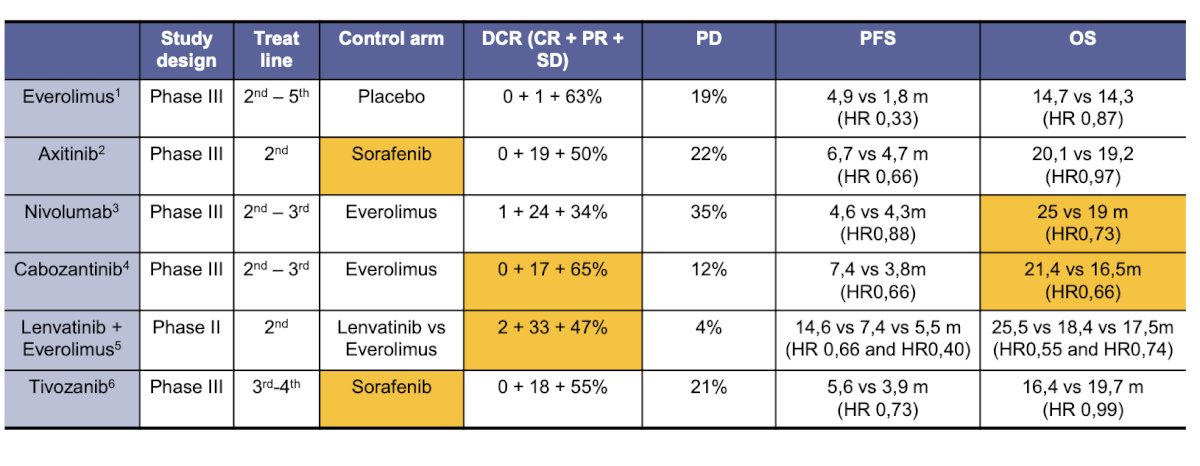
After TKI monotherapy for first-line mRCC, multiple phase III clinical trials are exploring Everolimus, Axitinib, Nivolumab, Cabozantinib, and Tivozanib monotherapy. Additionally, there is one phase II trial investigating the Lenvatinib and Everolimus combination. Two studies show a benefit in OS: one comparing Nivolumab versus Everolimus (median OS 15 vs. 19 months, HR 0.73) and the other comparing Cabozantinib versus Everolimus (median OS 21.1 vs. 16.5 months, HR 0.66).14,15
The next question to ask ourselves is how to rescue patients who had received IO+IO combinations or IO+VEGFR-TKI combinations. Multiple trials have looked at this question and are summarized in the table below. The ORR ranges from 14.5% to 45%, and the median PFS ranges from 5.6 months to 13.8 months. Notably, the most recent trial is the LITESPARK 003 cohort 2, exploring belzutifan plus cabozantinib after failure from treatment with IO+VEGFR-TKI. This phase II trial assigned patients with locally advanced or metastatic ccRCC previously treated with IO +/- VEGFR-TKI to belzutifan 120 mg orally once daily plus cabozantinib 60 mg orally once daily. The primary endpoint was investigator-determined ORR per RECIST v1.1. The investigators reported an ORR of 31%, with 4% achieving CR, and the median OS was 26.7 months.16 Based on these results, belzutifan was approved in the US for advanced RCC following a PD-(L)-1 inhibitor and a VEGFR-TKI.
Dr. Alonso Gordoa moved on to discuss the LITESPARK-005 phase III clinical trial (NCT04195750) presented at ESMO. In this trial, eligible patients were adults with mRCC who had received 1–3 prior systemic regimens, including at least one PD-(L)1 inhibitor and at least one VEGFR-TKI. Patients were randomized 1:1 to receive either belzutifan 120 mg (HIF-1/2 inhibitor) or everolimus 10 mg QD until disease progression or unacceptable adverse events. The trial design for LITESPARK-005 is shown below: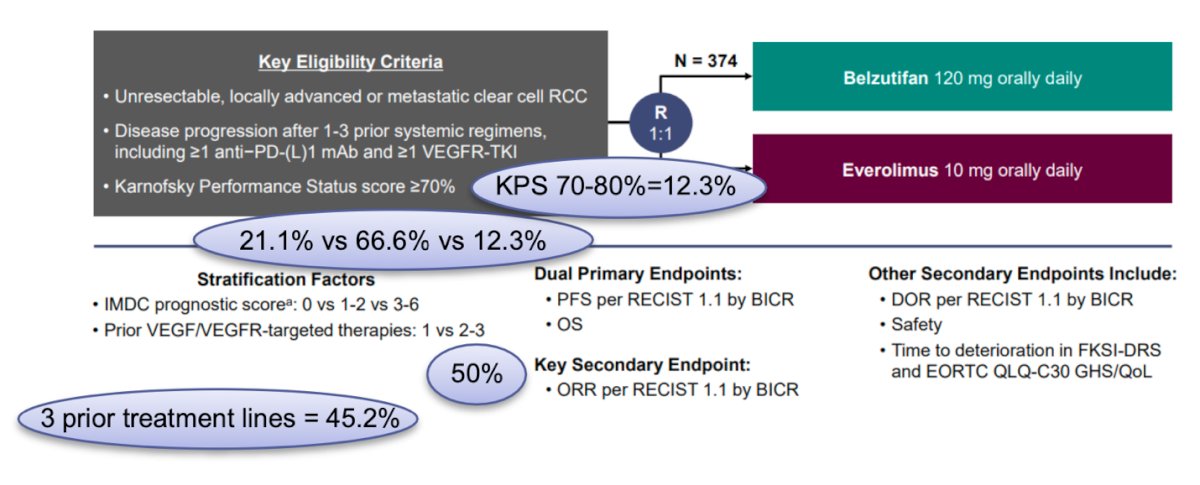
Notably, in the Belzutifan arm, 21.1%, 66.6%, and 12.3% of the patients had IMDC risk group, favorable, intermediate, and poor, 12.3% had a KPS of 12.3%, 45.2% had received three prior lines of treatment. With a median follow-up of 35.8 months, the PFS benefit was maintained with belzutifan vs everolimus (median 5.6 months versus 5.6 months; HR 0.75; 95% CI 0.63–0.88), with an estimated progression-free survival rate at 24 months (17.5% vs 4.1%) favoring belzutifan. Moreover, the median overall survival was 21.4 months with belzutifan versus 18.2 months with everolimus (HR 0.92; 95% CI 0.77–1.10; p = 0.18). The Kaplan-Meier figures are shown below: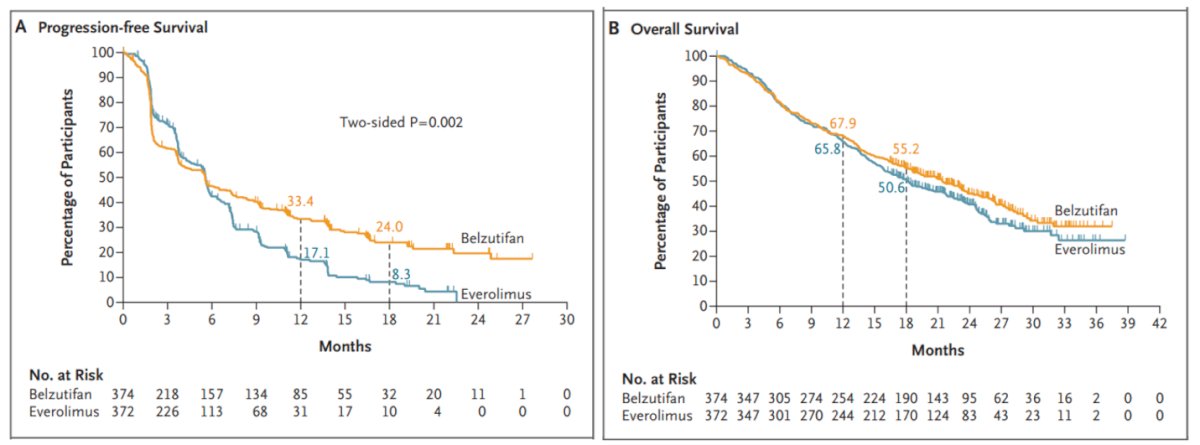
Dr. Alonso Gordoa discussed strategies for continuing IO-based treatments beyond progression, presenting data from the CONTACT-03 and TINIVO-2 trials. CONTACT-03 is notable as the first phase III randomized trial to test the benefit of immune checkpoint inhibitor rechallenge by directly adding it to a control arm. This trial evaluated the combination of anti–PD-L1 atezolizumab and the VEGFR-TKI cabozantinib versus cabozantinib alone in patients with mRCC who had progressed during or after prior IO treatment. Patients were randomized 1:1 to receive atezolizumab (1200 mg IV q3w) plus cabozantinib (60 mg oral QD) or cabozantinib alone. The median PFS was 11 months (in the second/third line), and the median OS was 26 months. However, the primary endpoint of PFS was not met, with an HR of 1.03 (95% CI 0.83–1.28; p=0.78).17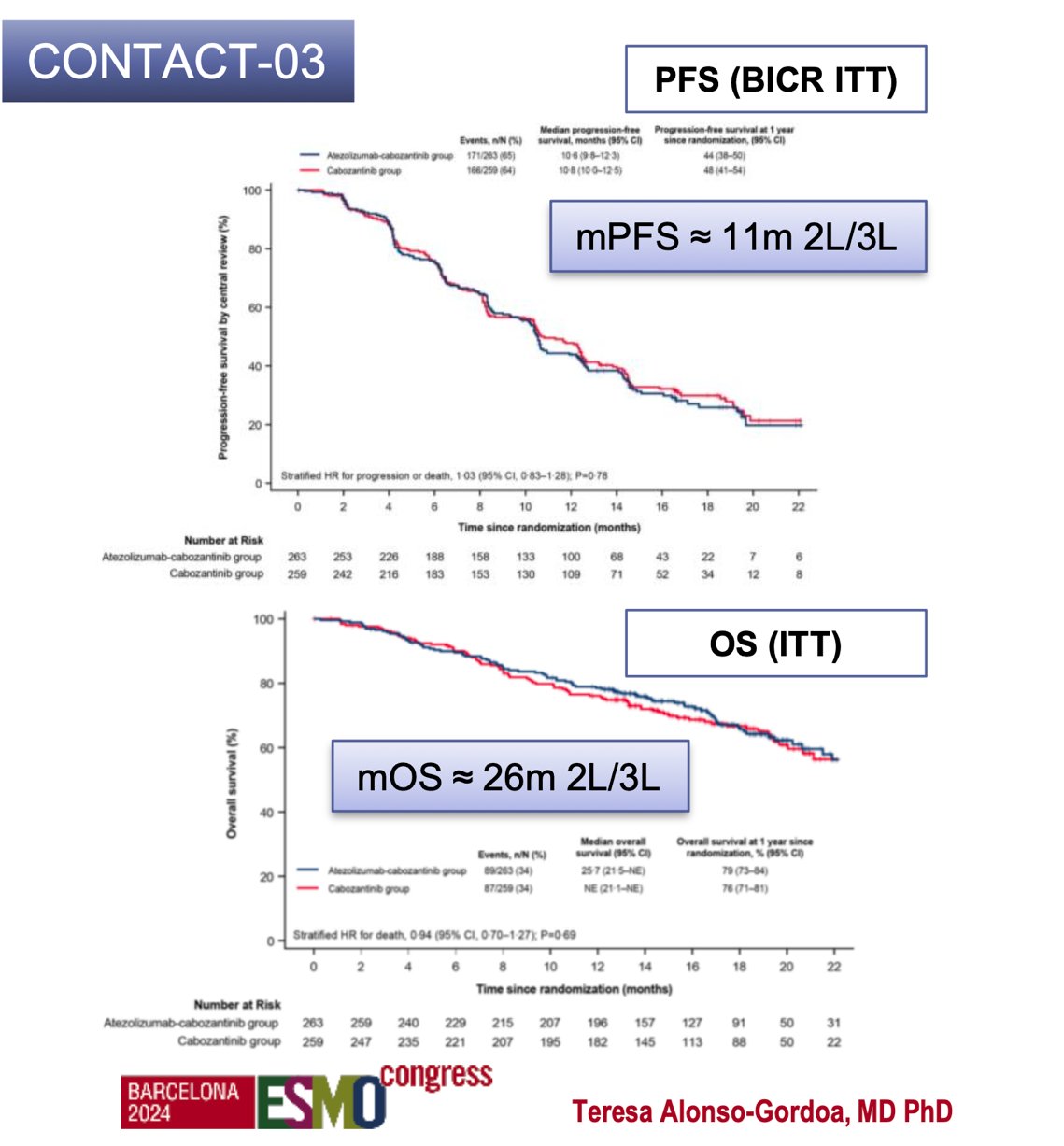
TiNivo-2 (NCT04987203) is a phase 3, randomized, controlled, multicenter, open-label study to compare tivozanib in combination with nivolumab to tivozanib monotherapy in subjects mRCC who have progressed following 1-2 lines of therapy including IO.18
Dr. Alonso Gordoa highlighted future efforts to improve therapy by targeting various mechanisms such as metabolism, epigenetics, the cell cycle, and angiogenesis. Additionally, other targets such as RB1, SMAD3, CDK4/6, and HIF should also be considered when designing clinical trials. She mentioned the LITESPARK-011 trial, which is evaluating Belzutifan plus Lenvatinib versus Cabozantinib in patients with mRCC and a clear cell component who progressed after first or second-line anti-PD1 or PD-L1 therapy, having received two or fewer lines of therapy.19
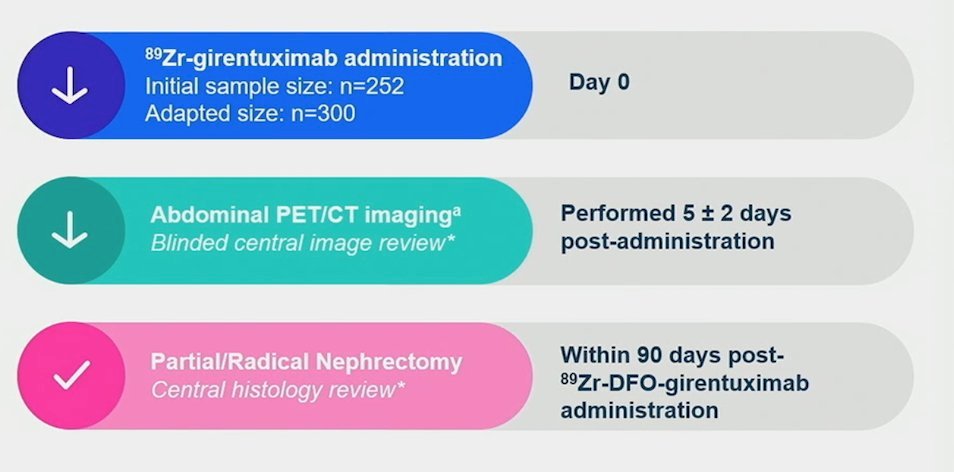
Future directions in ccRCC include the use of new radiotracers or potentially radioligand therapy. The ZIRCON trial evaluated Girentuximab, a monoclonal antibody that targets carbonic anhydrase IX, an enzyme highly expressed in ccRCC. Radiolabeled 89Zr-DFO-girentuximab (TLX250-CDx) is highly specific for carbonic anhydrase IX and can aid in differentiating between clear cell RCCs and other renal tumors. This open-label, multicenter clinical trial included patients with indeterminate renal masses (≤ 7 cm; tumor stage cT1) who were scheduled for partial nephrectomy. The enrolled patients received a single dose of TLX250-CDx IV (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery. The ZIRCON trial showed remarkable sensitivity and specificity of 86% (95% CI 80%-90%) and 87% (95% CI 79%-92%), respectively.20
The STARLITE-1 is a phase 1/2 trial is an investigator-initiated trial. enrolling patients with treatment-naïve ccRCC to receive 177Lu-girentuximab 1480 MBq/m2 (61% of single agent MTD) every 12 weeks for up to 3 treatment cycles. Starting with the second cycle, nivolumab, and cabozantinib will be added at standard dose. The STARLITE-2 trial is a phase 2 study of nivolumab plus 177Lutetium-labeled with girentuximab (177Lu-girentuximab) in patients with metastatic ccRCC.
Additionally, there are multiple new molecules being explored, including immunomodulatory probiotics, new cytokines, new immune checkpoint inhibitors (relatlimab, leramilimab, favezlimab), HIF inhibitors, and CAR-T cell therapy. All these therapeutic targets are shown in the graphic below. The future of RCC is promising.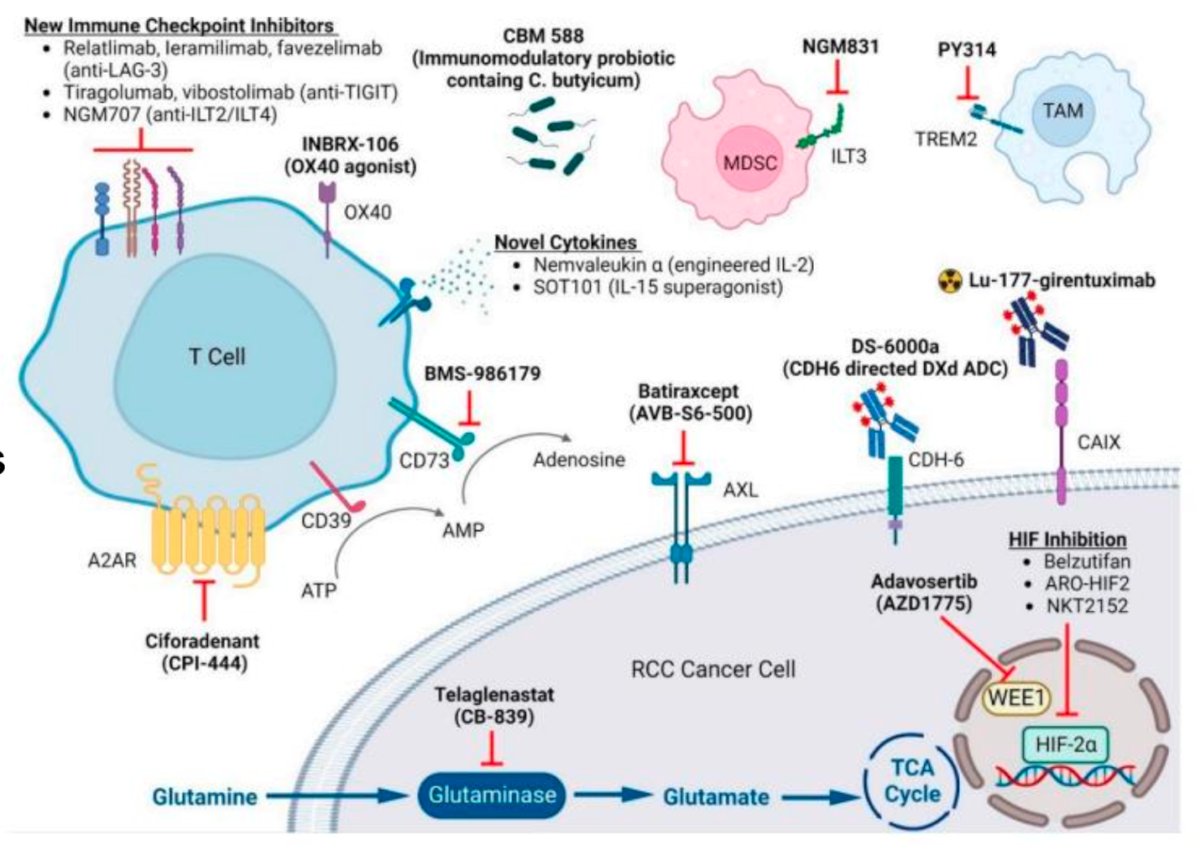
Non-clear cell Renal Cell Carcinoma
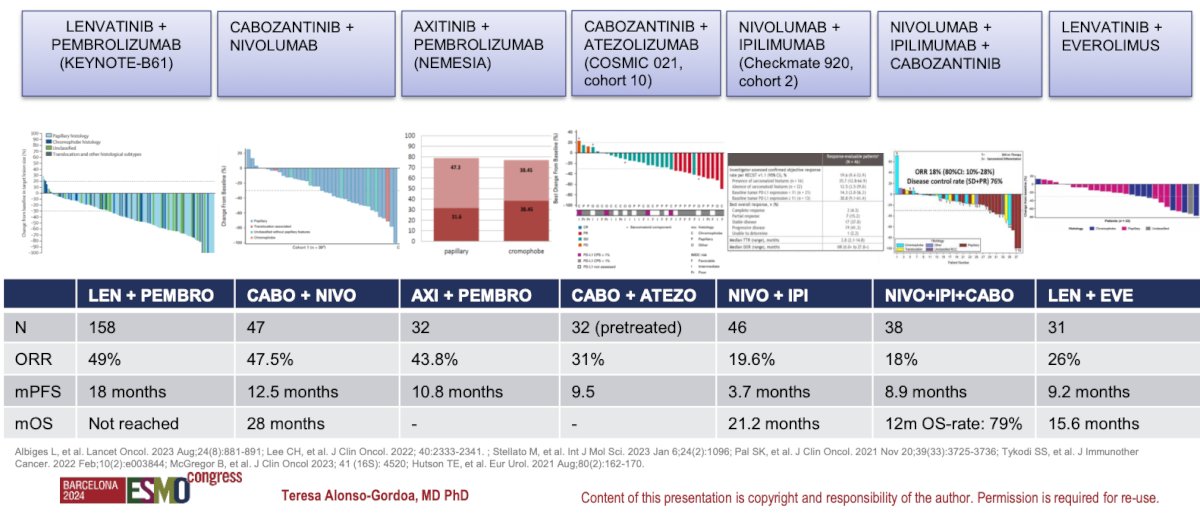
There are multiple prospective clinical trials that have compared different combinations of IO+IO, IO+VEGFR-TKI, and triplets of IO+IO+TKI. A comprehensive summary of the studies was presented by Dr. Alonso Gordoa and is summarized in the figure below.
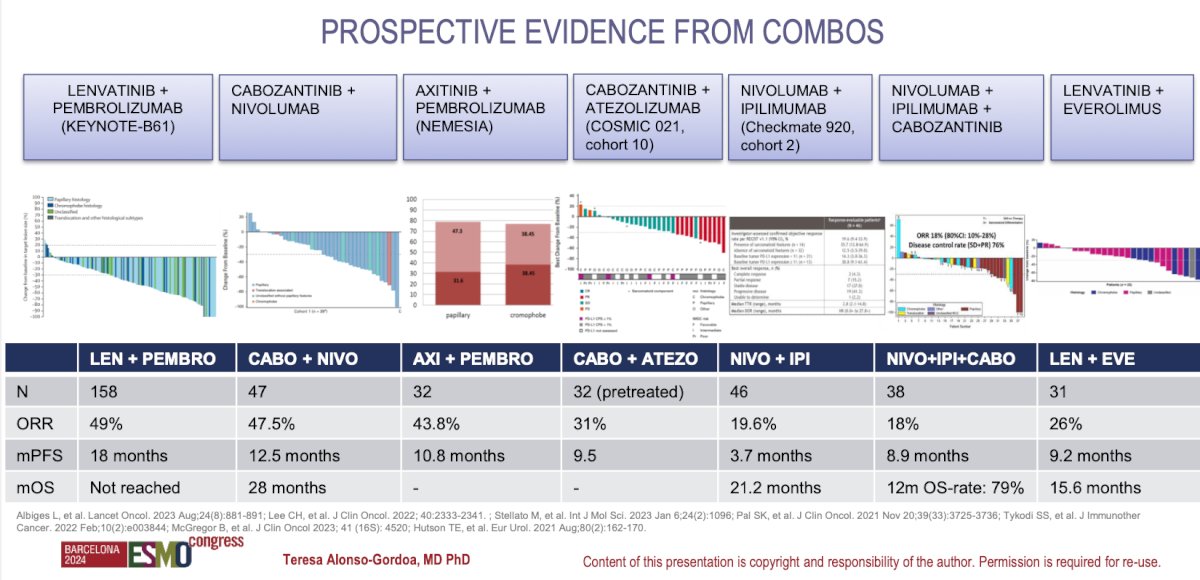
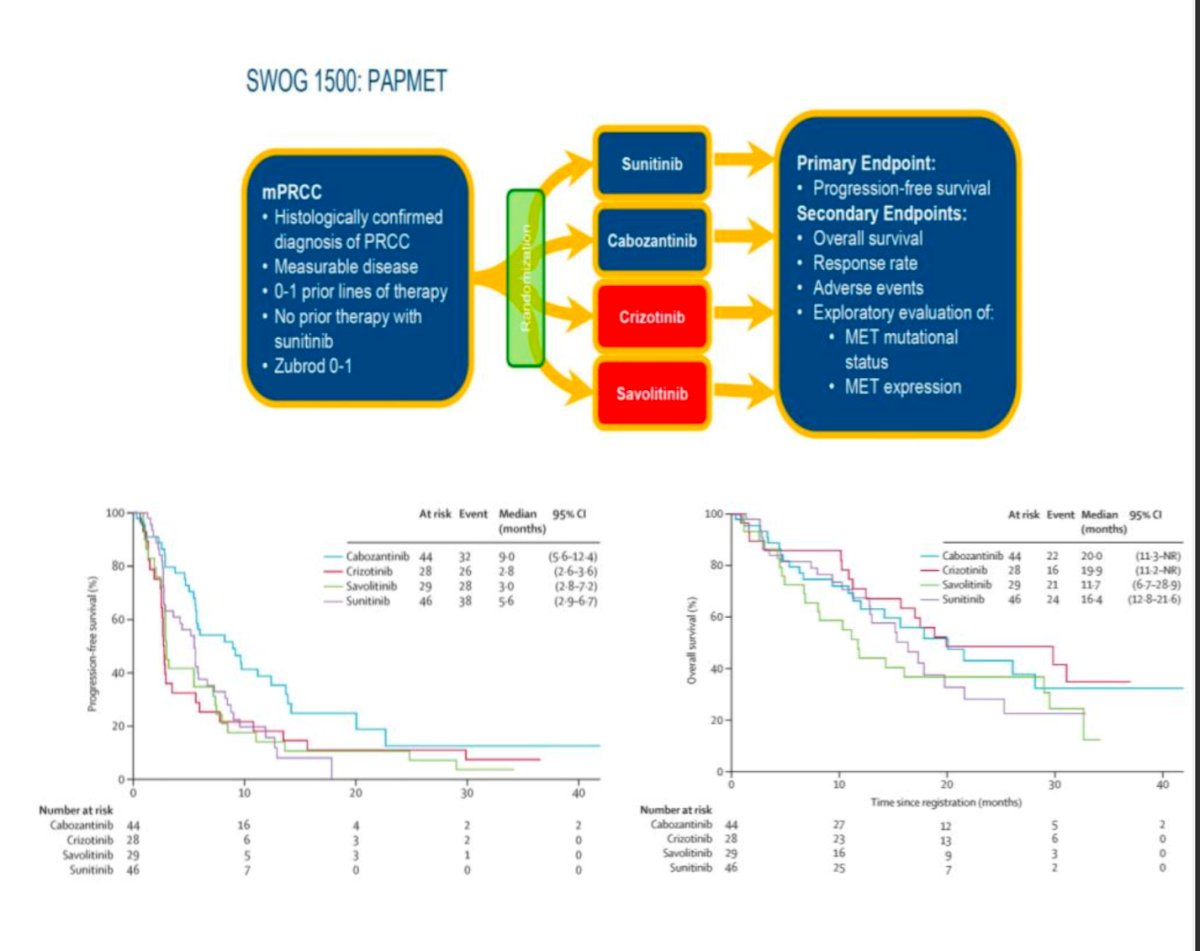
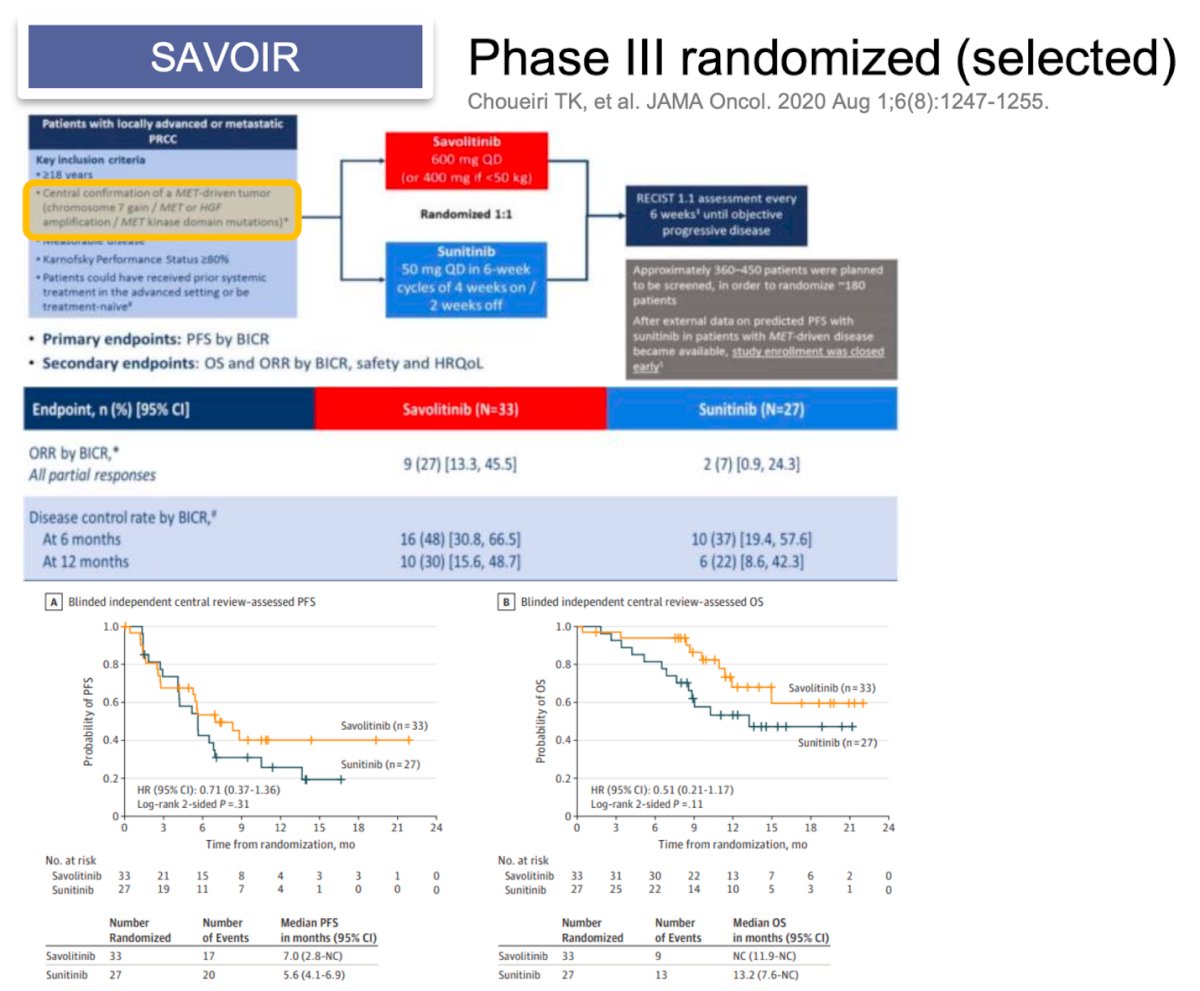
Dr. Alonso Gordoa presented the design of 6 clinical trials, five phase III and one phase II comparing different combinations for non-clear cell RCC. The first trial was the SAVOIR, this is a phase III randomized trial including patients with confirmation of. MET-driven tumor (chromosome 7 gain, MET or HGF amplification, and MET kinase domain mutations) and randomized them to Savolitinib vs. Sunitinib the primary endpoint of PFS was not met (HR 0.71 95% CI0.37-1.36). However, the ORR was 27% with Savolitinib vs 7% with Sunitinib.
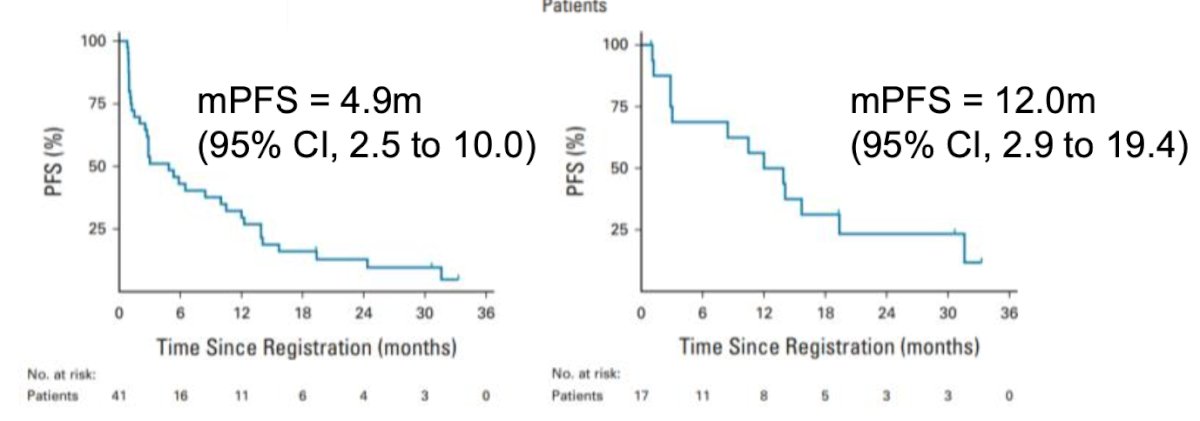
The CALYPSO trial was a phase II single-arm trial involving patients with metastatic papillary RCC (unselected) treated with savolitinib plus durvalumab. The CR rate was 29%, and the primary endpoint was not met. However, the CR increased to 53% in MET-driven patients (N = 9/27). Additionally, the median PFS was 4.9 months in the treated population and 12.0 months in MET-driven patients, highlighting the importance of selecting patients with non-clear cell RCC with MET alterations for therapy with savolitinib.21
Dr. Alonso Gordoa presented the design of the remaining four trials, which mostly included patients with papillary RCC. The details of these trials are summarized in the slide below.

Lastly, she discussed various combinations for patients with RCC and sarcomatoid differentiation. An exploratory analysis of CheckMate 214 compared Nivolumab + Ipilimumab versus Sunitinib in patients with sarcomatoid differentiation.7 The results demonstrated an ORR of 60.8% versus 23.1%, a CR rate of 23% versus 6.2%, and a significant median OS favoring the combination therapy (48.6 versus 14.2 months, HR 0.46).
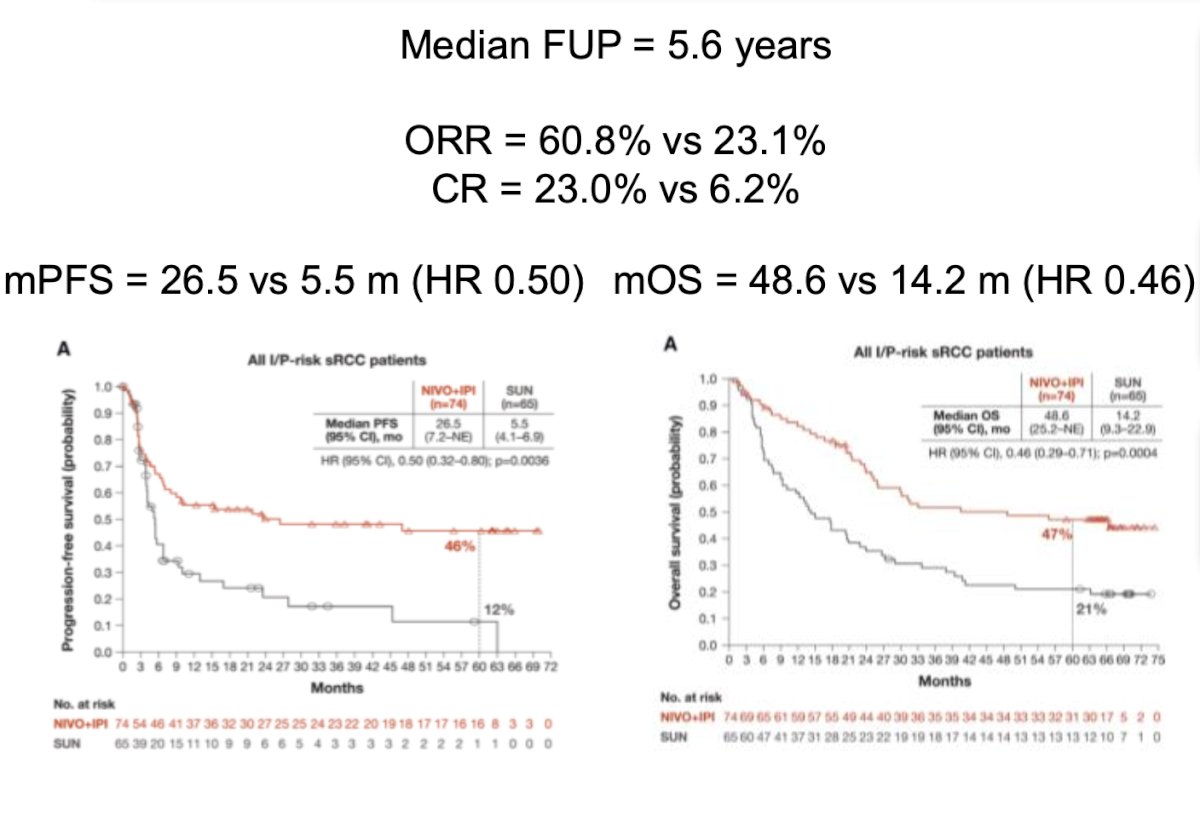
Furthermore, in the KEYNOTE 426, CLEAR, and CheckMate 9ER trials, the combinations have demonstrated significant improvements in ORR, median PFS, and OS for patients with sarcomatoid differentiation. The results are summarized below:
Dr. Alonso Gordoa concluded her presentation with the following remarks:
- IO-IO and IO-TKI combinations are foundational in the first-line treatment for advanced RCC.
- Differences in patient populations, efficacy outcomes, and QoL should be considered when selecting treatments across studies.
- These strategies enhance response rates and survival, potentially allowing for therapy interruptions or de-escalation approaches to improve tolerability, in collaboration with a multidisciplinary team.
- Ongoing research is exploring new combinations and strategies in treatment sequencing through clinical trials.
- There is an urgent need for biomarkers and advancements in molecular biology to guide clinical decision-making (CARE 1).
Presented by: Teresa Alonso Gordoa, MD, Medical Oncologist · Hospital Universitario Ramón y Cajal, Madrid, Spain.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999 Aug;17(8):2530-40. doi: 10.1200/JCO.1999.17.8.2530. PMID: 10561319.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B; CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018 Apr 5;378(14):1277-1290. doi: 10.1056/NEJMoa1712126. Epub 2018 Mar 21. PMID: 29562145; PMCID: PMC5972549.
- Plimack ER, Powles T, Stus V, Gafanov R, Nosov D, Waddell T, Alekseev B, Pouliot F, Melichar B, Soulières D, Borchiellini D, McDermott RS, Vynnychenko I, Chang YH, Tamada S, Atkins MB, Li C, Perini R, Molife LR, Bedke J, Rini BI. Pembrolizumab Plus Axitinib Versus Sunitinib as First-line Treatment of Advanced Renal Cell Carcinoma: 43-month Follow-up of the Phase 3 KEYNOTE-426 Study. Eur Urol. 2023 Nov;84(5):449-454. doi: 10.1016/j.eururo.2023.06.006. Epub 2023 Jul 25. Erratum in: Eur Urol. 2023 Nov;84(5):e123-e124. doi: 10.1016/j.eururo.2023.08.010. Erratum in: Eur Urol. 2024 Feb;85(2):e58-e59. doi: 10.1016/j.eururo.2023.11.016. PMID: 37500340.
- Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, Méndez-Vidal MJ, Tjulandin S, Hoon Park S, Melichar B, Hutson T, Alemany C, McGregor B, Powles T, Grünwald V, Alekseev B, Rha SY, Kopyltsov E, Kapoor A, Alonso Gordoa T, Goh JC, Staehler M, Merchan JR, Xie R, Perini RF, Mody K, McKenzie J, Porta CG. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023 Mar;24(3):228-238. doi: 10.1016/S1470-2045(23)00049-9. Erratum in: Lancet Oncol. 2023 Apr;24(4):e146. doi: 10.1016/S1470-2045(23)00117-1. PMID: 36858721.
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suárez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Żołnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ; CheckMate 9ER Investigators. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021 Mar 4;384(9):829-841. doi: 10.1056/NEJMoa2026982. PMID: 33657295; PMCID: PMC8436591.
- Choueiri TK, Powles T, Albiges L, Burotto M, Szczylik C, Zurawski B, Yanez Ruiz E, Maruzzo M, Suarez Zaizar A, Fein LE, Schutz FA, Heng DYC, Wang F, Mataveli F, Chang YL, van Kooten Losio M, Suarez C, Motzer RJ; COSMIC-313 Investigators. Cabozantinib plus Nivolumab and Ipilimumab in Renal-Cell Carcinoma. N Engl J Med. 2023 May 11;388(19):1767-1778. doi: 10.1056/NEJMoa2212851. PMID: 37163623; PMCID: PMC10257898.
- Brown JE, Royle KL, Gregory W, Ralph C, Maraveyas A, Din O, Eisen T, Nathan P, Powles T, Griffiths R, Jones R, Vasudev N, Wheater M, Hamid A, Waddell T, McMenemin R, Patel P, Larkin J, Faust G, Martin A, Swain J, Bestall J, McCabe C, Meads D, Goh V, Min Wah T, Brown J, Hewison J, Selby P, Collinson F; STAR Investigators. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023 Mar;24(3):213-227. doi: 10.1016/S1470-2045(22)00793-8. Epub 2023 Feb 13. PMID: 36796394.
- Mantia CM, Jegede OA, Plimack ER, Powles T, Motzer RJ, Tannir NM, Lee CH, Tomita Y, Voss MH, Choueiri TK, Rini BI, Hammers HJ, Escudier B, Albigès L, Rosenblatt L, Atkins MB, Regan MM, McDermott DF. Treatment-free survival and partitioned survival analysis of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib: 5-year update of CheckMate 214. J Immunother Cancer. 2024 Jul 25;12(7):e009495. doi: 10.1136/jitc-2024-009495. PMID: 39060019; PMCID: PMC11284827.
- Atkins MB, Jegede OA, Haas NB, McDermott DF, Bilen MA, Stein M, Sosman JA, Alter R, Plimack ER, Ornstein M, Hurwitz M, Peace DJ, Signoretti S, Denize T, Cimadamore A, Wu CJ, Braun D, Einstein D, Catalano PJ, Hammers H. Phase II Study of Nivolumab and Salvage Nivolumab/Ipilimumab in Treatment-Naive Patients With Advanced Clear Cell Renal Cell Carcinoma (HCRN GU16-260-Cohort A). J Clin Oncol. 2022 Sep 1;40(25):2913-2923. doi: 10.1200/JCO.21.02938. Epub 2022 Apr 20. PMID: 35442713; PMCID: PMC9426835.
- Iacovelli R, Ciccarese C, Buti S, Zucali PA, Fantinel E, Bimbatti D, Verzoni E, Accettura C, Bonomi L, Buttigliero C, Fornarini G, Pipitone S, Atzori F, Masini C, Massari F, Primi F, Strusi A, Giudice GC, Perrino M, Maruzzo M, Milella M, Giannarelli D, Brunelli M, Procopio G, Tortora G. Avelumab Plus Intermittent Axitinib in Previously Untreated Patients with Metastatic Renal Cell Carcinoma. The Tide-A Phase 2 Study. Eur Urol. 2024 Mar 22:S0302-2838(24)02132-8. doi: 10.1016/j.eururo.2024.02.014. Epub ahead of print. PMID: 38521617.
- McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, Wiechno P, Gafanov RA, Tomczak P, Pouliot F, Donskov F, Alekseev BY, Shin SJ, Bjarnason GA, Castellano D, Silverman RK, Perini RF, Schloss C, Atkins MB. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2021 Mar 20;39(9):1029-1039. doi: 10.1200/JCO.20.02365. Epub 2021 Feb 2. PMID: 33529058; PMCID: PMC8078262.
- Choueiri TK, McDermott DF, Merchan J, Bauer TM, Figlin R, Heath EI, Michaelson MD, Arrowsmith E, D'Souza A, Zhao S, Roy A, Perini R, Vickery D, Tykodi SS. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: an open-label, single-arm, phase 2 study. Lancet Oncol. 2023 May;24(5):553-562. doi: 10.1016/S1470-2045(23)00097-9. Epub 2023 Mar 31. PMID: 37011650.
- Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Chang YH, Lee JL, Sarwar N, Haas NB, Gurney H, Sawrycki P, Mahave M, Gross-Goupil M, Zhang T, Burke JM, Doshi G, Melichar B, Kopyltsov E, Alva A, Oudard S, Topart D, Hammers H, Kitamura H, McDermott DF, Silva A, Winquist E, Cornell J, Elfiky A, Burgents JE, Perini RF, Powles T; KEYNOTE-564 Investigators. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024 Apr 18;390(15):1359-1371. doi: 10.1056/NEJMoa2312695. PMID: 38631003.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015 Nov 5;373(19):1803-13. doi: 10.1056/NEJMoa1510665. Epub 2015 Sep 25. PMID: 26406148; PMCID: PMC5719487.
- Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Géczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ; METEOR Investigators. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015 Nov 5;373(19):1814-23. doi: 10.1056/NEJMoa1510016. Epub 2015 Sep 25. PMID: 26406150; PMCID: PMC5024539.
- Choueiri TK, McDermott DF, Merchan J, Bauer TM, Figlin R, Heath EI, Michaelson MD, Arrowsmith E, D'Souza A, Zhao S, Roy A, Perini R, Vickery D, Tykodi SS. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: an open-label, single-arm, phase 2 study. Lancet Oncol. 2023 May;24(5):553-562. doi: 10.1016/S1470-2045(23)00097-9. Epub 2023 Mar 31. PMID: 37011650.
- Pal SK, Albiges L, Tomczak P, Suárez C, Voss MH, de Velasco G, Chahoud J, Mochalova A, Procopio G, Mahammedi H, Zengerling F, Kim C, Osawa T, Angel M, Gupta S, Khan O, Bergthold G, Liu B, Kalaitzidou M, Huseni M, Scheffold C, Powles T, Choueiri TK. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2023 Jul 15;402(10397):185-195. doi: 10.1016/S0140-6736(23)00922-4. Epub 2023 Jun 5. PMID: 37290461; PMCID: PMC11017728.
- Toni K. Choueiri et al.TiNivo-2: A phase 3, randomized, controlled, multicenter, open-label study to compare tivozanib in combination with nivolumab to tivozanib monotherapy in subjects with renal cell carcinoma who have progressed following one or two lines of therapy where one line has an immune checkpoint inhibitor. JCO 40, TPS405-TPS405(2022).
- Motzer RJ, Schmidinger M, Eto M, Suarez C, Figlin R, Liu Y, Perini R, Zhang Y, Heng DY. LITESPARK-011: belzutifan plus lenvatinib vs cabozantinib in advanced renal cell carcinoma after anti-PD-1/PD-L1 therapy. Future Oncol. 2023 Jan;19(2):113-121. doi: 10.2217/fon-2022-0802. Epub 2023 Feb 8. PMID: 36752726.
- Shuch B, Pantuck AJ, Bernhard JC, Morris MA, Master V, Scott AM, van Praet C, Bailly C, Önal B, Aksoy T, Merkx R, Schuster DM, Lee ST, Pandit-Taskar N, Fan AC, Allman P, Schmidt K, Tauchmanova L, Wheatcroft M, Behrenbruch C, Hayward CRW, Mulders P. [89Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: a prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024 Sep 10:S1470-2045(24)00402-9. doi: 10.1016/S1470-2045(24)00402-9. Epub ahead of print. PMID: 39270701.
- Hasanov E, Flynt L, Slack Tidwell R, Hwang H, Brooks R, King L, Solley T, Syed M, Yamamura Y, Hayward C, Venkatesan AM, Jonasch E. STARLITE 1: Phase 1b/2 study of combination 177Lu girentuximab plus cabozantinib and nivolumab in treatment naïve patients with advanced clear cell RCC. Oncologist. 2023 Aug 23;28(Suppl 1):S13. doi: 10.1093/oncolo/oyad216.021. PMCID: PMC10445563.
- Suárez C, Larkin JMG, Patel P, Valderrama BP, Rodriguez-Vida A, Glen H, Thistlethwaite F, Ralph C, Srinivasan G, Mendez-Vidal MJ, Hartmaier R, Markovets A, Prendergast A, Szabados B, Mousa K, Powles T. Phase II Study Investigating the Safety and Efficacy of Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer (CALYPSO). J Clin Oncol. 2023 May 10;41(14):2493-2502. doi: 10.1200/JCO.22.01414. Epub 2023 Feb 21. Erratum in: J Clin Oncol. 2023 Aug 10;41(23):3961. doi: 10.1200/JCO.23.01150. PMID: 36809050.


