(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the presentation of Poster 1701. Dr. Liangyou Gu presented the results of NEOTAX: a Phase II trial of neoadjuvant toripalimab plus axitinib for clear cell renal cell carcinoma with inferior vena cava (IVC) tumor thrombus.
Renal cell carcinoma (RCC) extends into the renal vein and inferior vena cava (IVC) in up to 25% of patients. Despite advances in medical management, such as targeted therapy and immunotherapy for advanced disease, surgical resection remains the mainstay treatment for RCC with venous tumor thrombus (TT) extension and is the only hope for a potential cure. However, radical nephrectomy with IV thrombectomy has historically been associated with high surgical morbidity and mortality.
Preoperative TT regression and tumor shrinkage may reduce surgical morbidity and perioperative complications. A recent systematic review assessing preoperative targeted therapy and immunotherapy-based combination therapy in patients with RCC and IVC TT extension showed that the use of neoadjuvant therapy may be associated with a total thrombus level reduction rate of 29.4% and is linked to shorter operative time (p<0.05) and less blood loss (p<0.05).1,2
RENOTORCH is a phase 3 trial that evaluated the efficacy of the combination toripalimab and axitinib in metastatic RCC versus sunitinib, the combination demonstrated an objective response rate (ORR0 of 56.7% compared to 30.8% in the sunitinib arm.3
NEOTAX (ChiCTR 2000030405) was a single-arm, interventional, phase 2 study of neoadjuvant Toripalimab (Tuoyi™) a selective, recombinant, humanized monoclonal antibody against programmed death protein 1 (PD-1) in combination with axitinib (VEGF inhibitor) in patients with clear cell renal cell carcinoma (ccRCC) and IVC-TT (Mayo level Ⅱ-Ⅳ). Toripalimab was administered at 240 mg every 3 weeks for up to 4 cycles. The dose of axitinib was 5 mg BID. The primary endpoint was the down-staging rate of IVC-TT level. Secondary endpoints were percentage change in surgical approach and TT length, response rate (RECISTv1.1), progression-free survival, surgical morbidity, and biomarker exploration. The study design is illustrated in the diagram below:
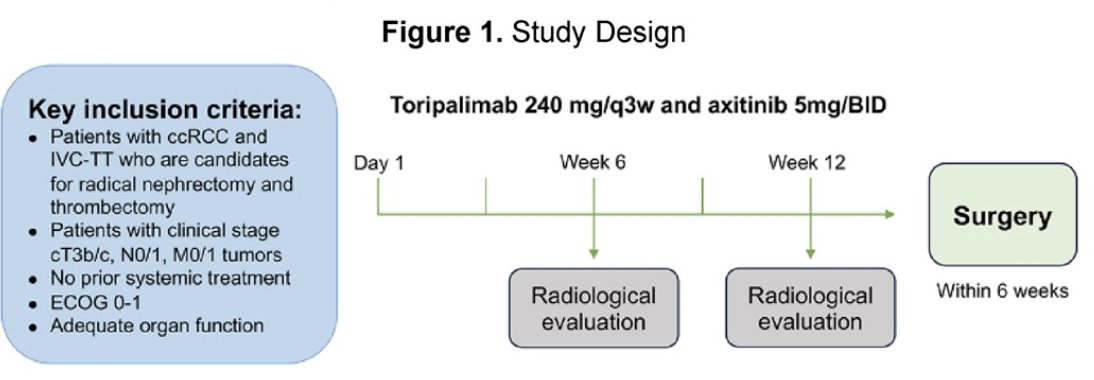
Twenty-nine patients with clear cell RCC and level II to IV IVC thrombus (cT3b/c), cN0/1, CM0/1, who were candidates for radical nephrectomy and IVC thrombectomy, were enrolled in the trial. A total of 25 patients received the study treatment. The median age was 58 years (IQR, 51.5-67.5 years), and most patients were male (76.0%). Ten patients (40.0%) had lymph node metastasis, and five patients (25.0%) had distant metastasis. The table below depicts the baseline clinical characteristics.
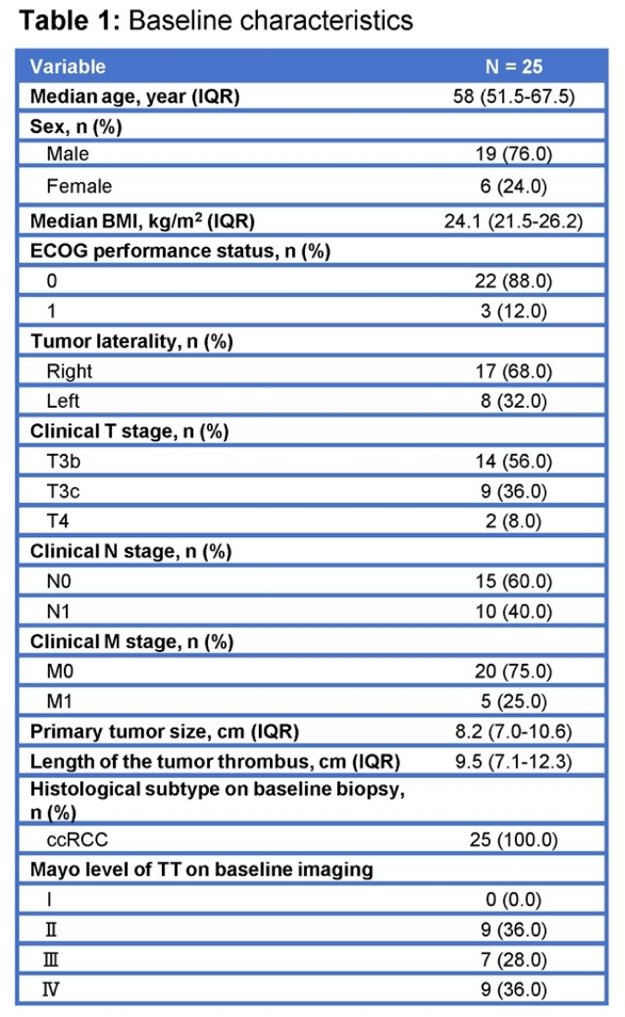
After 12 weeks of treatment. 44% (11/25) patients experienced a reduction in TT level. A total of 24/25 (96.0%) patients had any reduction in TT length (range 5-79%), the median change in tumor thrombus length was -2.3 cm (range: -7.1 to 1.1 cm).
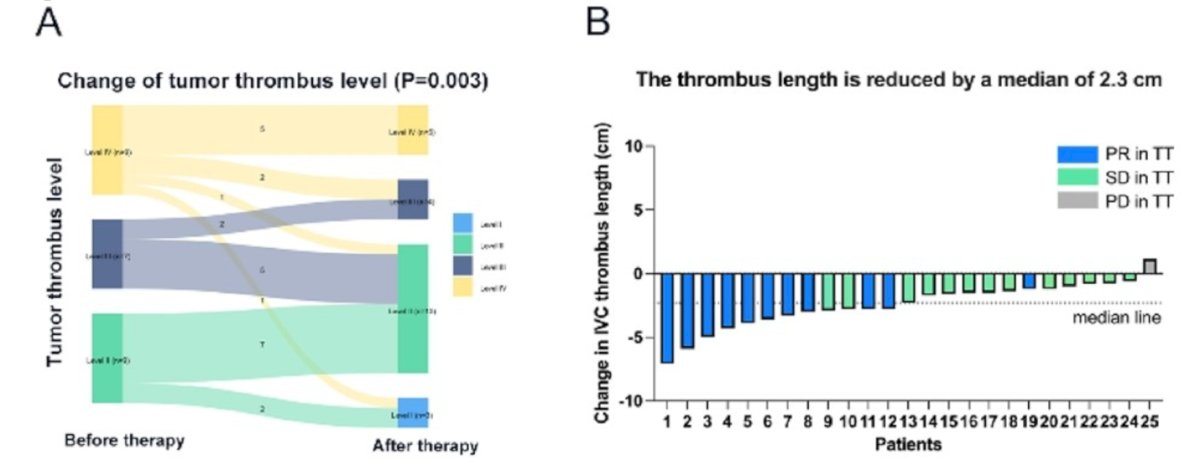
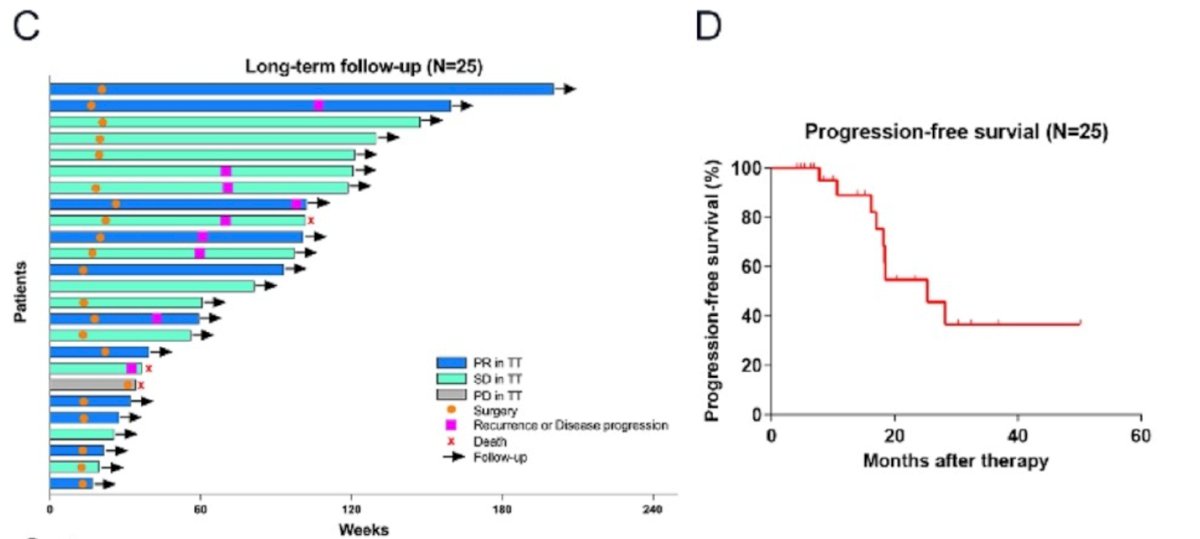
Tumor progression occurred in 9 patients (36.0%), with 7 of them progressing after nephrectomy and IVC thrombectomy. The 1-year and 2-year progression-free survival (PFS) rates were 89.1% and 54.8%, respectively.
Eighty-one percent of the surgeries were performed using laparoscopic robot-assisted surgery, while 19% were open. Of the surgeries, 71.4% included thrombectomy and 28.5% required cavectomy. A total of 61.9% (13/21) of patients experienced changes in the surgical strategy compared with the planned surgery. Postoperative complications occurred in 57.1% (12/21) of patients. Other perioperative details are shown in the table below:

There were no grade 4/5 treatment-related adverse events. Overall, grade 3 AEs occurred in seven patients (28%). The most common grade 3 TRAEs were hypertension (8%), and proteinuria (8%).
Dr. Gu and colleagues analyzed the biopsy samples of all patients, and they discovered that biopsy samples of non-responders exhibited increased T cytotoxic cell infiltration, but these cells were predominantly PD-1 positive. Biopsy samples of responders to therapy exhibited lower T helper cells, however, their subtype, regulatory T cells remained unchanged. In surgical samples of the TT, non-responders exhibited increased CD8T_01_GZMK_CXCR4 subset T cells.
Dr. Gu concluded their poster with the following takeaway messages:
- NEOTAX provides the first level II evidence that toripalimab in combination with axitinib can downstage IVC-TT in a significant proportion of patients.
-44% in tumor thrombus level
-96% in tumor thrombus length
- The median change in tumor thrombus length was -2.3 cm, leading to a reduction in the extent of surgery and leading to changes in the surgical strategy in up to 62% of cases.
- Biopsy samples of non-responders exhibited increased T cytotoxic cell infiltration, while responders showed lower T helper cells, warranting further research into the tumor microenvironment and response to anti-PD1 and VEGF inhibitors
Presented by: Liangyou Gu, Department of Urology, The Third Medical Centre, Chinese PLA General Hospital, Beijing, China.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Margulis V, Freifeld Y, Pop LM, Manna S, Kapur P, Pedrosa I, Christie A, Mohamad O, Mannala S, Singla N, Wait M, Bagrodia A, Woldu SL, Gahan J, Brugarolas J, Timmerman R, Hannan R. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus-Safety Lead-in Results of a Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2021 Jul 15;110(4):1135-1142. doi: 10.1016/j.ijrobp.2021.01.054. Epub 2021 Feb 5. PMID: 33549705; PMCID: PMC8856732.
- Gu L, Peng C, Li H, Jia T, Chen X, Wang H, Du S, Tang L, Liang Q, Wang B, Ma X, Zhang X. Neoadjuvant therapy in renal cell carcinoma with tumor thrombus: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2024 Apr;196:104316.
- Yan XQ, Ye MJ, Zou Q, Chen P, He ZS, Wu B, He DL, He CH, Xue XY, Ji ZG, Chen H, Zhang S, Liu YP, Zhang XD, Fu C, Xu DF, Qiu MX, Lv JJ, Huang J, Ren XB, Cheng Y, Qin WJ, Zhang X, Zhou FJ, Ma LL, Guo JM, Ding DG, Wei SZ, He Y, Guo HQ, Shi BK, Liu L, Liu F, Hu ZQ, Jin XM, Yang L, Zhu SX, Liu JH, Huang YH, Xu T, Liu B, Sun T, Wang ZJ, Jiang HW, Yu DX, Zhou AP, Jiang J, Luan GD, Jin CL, Xu J, Hu JX, Huang YR, Guo J, Zhai W, Sheng XN. Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: RENOTORCH, a randomized, open-label, phase III study. Ann Oncol. 2024 Feb;35(2):190-199. doi: 10.1016/j.annonc.2023.09.3108. Epub 2023 Oct 21. PMID: 37872020.


