- Eight abstracts, including two oral presentations, feature new clinical data from Astellas’ oncology portfolio and two lead pipeline programs across a broad range of cancer types
Tadaaki Taniguchi, MD, PhD, Chief Medical Officer, Astellas:
“The data presented at ESMO are an exciting demonstration of the strength of our portfolio and the transformative potential of our pipeline to help deliver outcomes that matter for patients. Astellas has made a long-term commitment to helping people living with hard-to-treat cancers by both investing in next-generation modalities like targeted protein degradation and immuno-oncology, and by maximizing the number of patients that could benefit from our approved medicines.”Highlights at the ESMO Congress 2024 include:
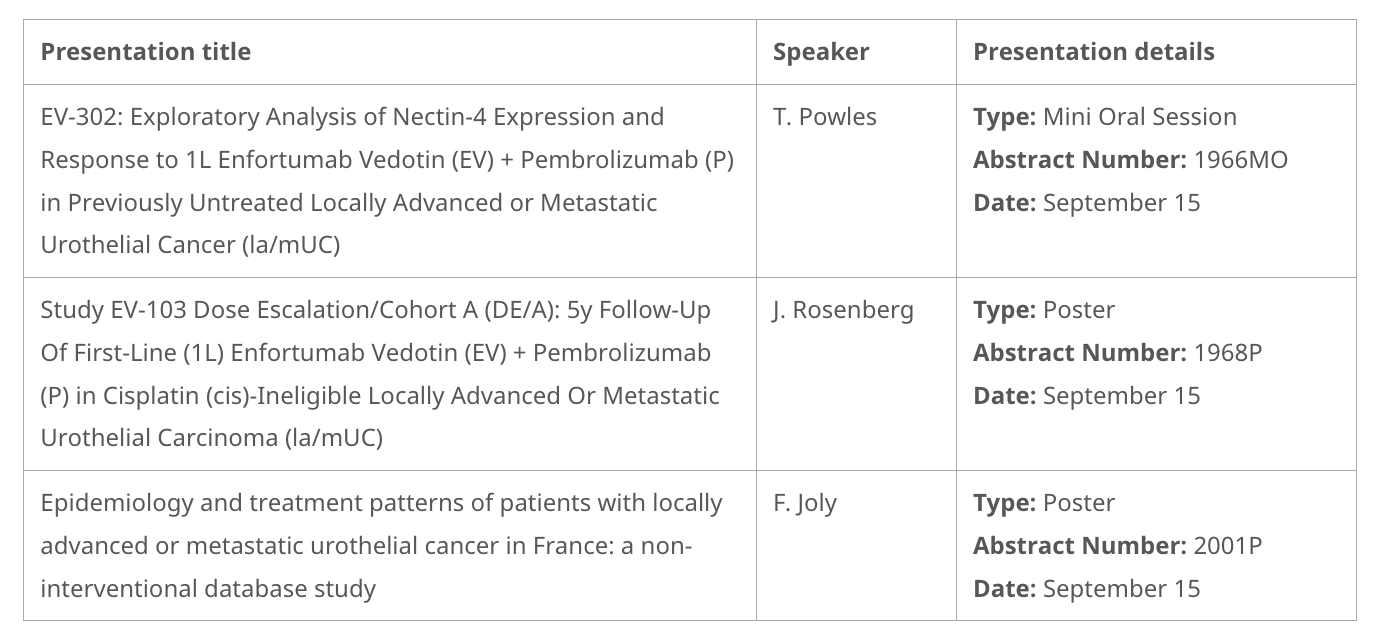
- Data from the Phase 3 EV-302 study, evaluating Nectin-4 expression and response to first-line treatment of enfortumab vedotin in combination with pembrolizumab in previously untreated locally advanced or metastatic urothelial cancer (la/mUC). These results support the combination as a first-line advancement across la/mUC patient subgroups, regardless of Nectin-4 expression.
- Five-year follow-up data from the Phase 1/2b EV-103 DE/A study, analyzing durable responses and meaningful survival to enfortumab vedotin in combination with pembrolizumab in patients with first-line cis-ineligible la/mUC. These results further support the broad suitability, regardless of cis-eligibility, and long-term benefits of this regimen in la/mUC.
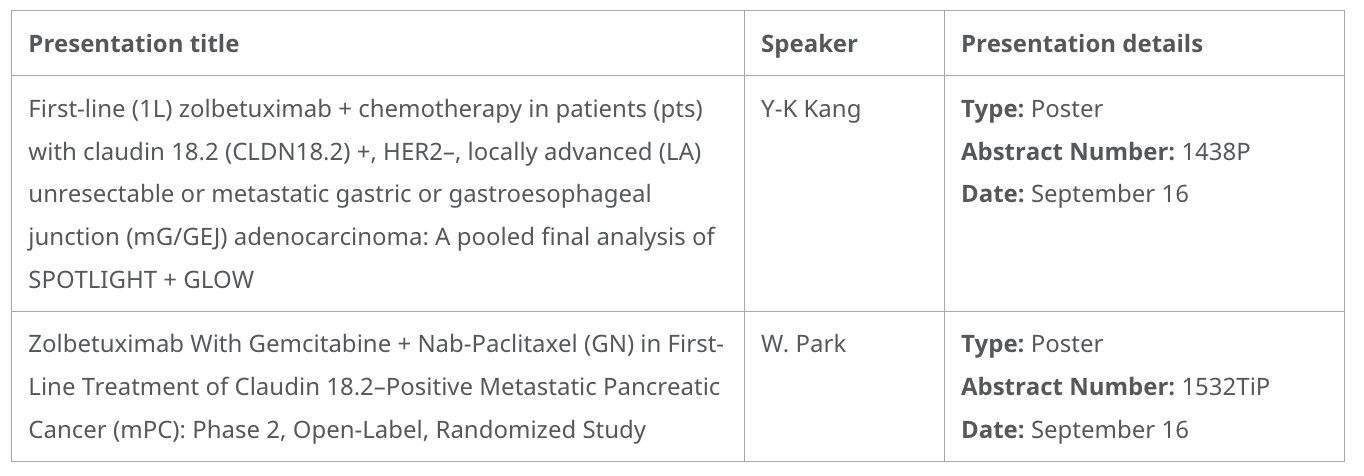
- Final pooled overall survival data from the Phase 3 SPOTLIGHT and GLOW trials, evaluating zolbetuximab plus chemotherapy as a first-line treatment in patients with HER2-negative, locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma whose tumors are Claudin 18.2-positive.
- Updates involving a Phase 2 trial in progress assessing zolbetuximab in combination with gemcitabine and nab-paclitaxel (GN) versus GN monotherapy as first-line treatment of Claudin 18.2-positive metastatic pancreatic cancer.
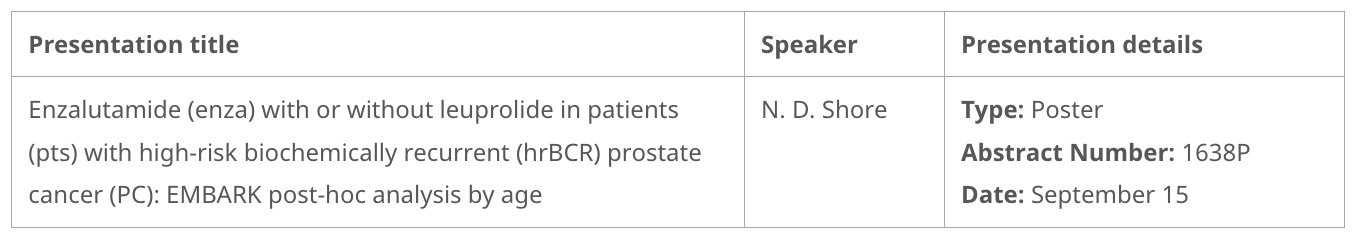
- Phase 3 EMBARK post-hoc analyses, evaluating enzalutamide in combination with leuprolide and as monotherapy versus leuprolide alone in patients with high-risk biochemical recurrent non-metastatic hormone-sensitive prostate cancer. These results support the benefits of enzalutamide both in combination with leuprolide and as monotherapy in patients aged <70 and ≥70 years.
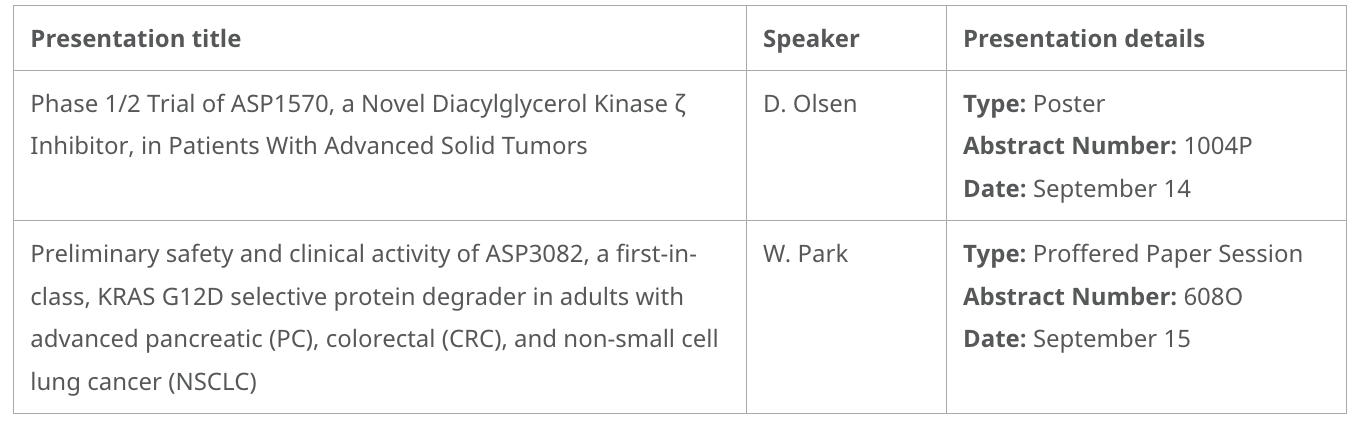
- Clinical data from lead pipeline assets: Phase 1 data from ASP3082, the first protein degrader targeting KRAS G12D mutant to enter clinical trials, in patients with advanced pancreatic, colorectal, and non-small cell lung cancer; and preclinical, translational/early clinical data from ASP1570, a novel DGKζ inhibitor, in patients with advanced solid tumors. The results support continued study of these investigational therapies for the treatment of various cancer types.
Astellas Presentations at 2024 ESMO Congress
Enfortumab Vedotin
Zolbetuximab
Enzalutamide
Pipeline
Source: © Astellas Pharma Inc. (2024 Sept 11). Astellas Presents Scientific Progress in Advanced and Hard-to-Treat Cancers at ESMO 2024 [Press release]. https://www.astellas.com/en/news/29431


