(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the session Therapeutic options beyond AR pathway inhibitors: What do we choose next? Dr. Elena Castro discussed if cytotoxics are still the next option after ARPIs.
Dr. Castro began by highlighting the significant changes in the treatment landscape for prostate cancer (PCa) in recent years. A wide array of systemic therapies has shown benefits in prolonging survival and has been approved for the treatment of PCa, thus expanding the treatment armamentarium.
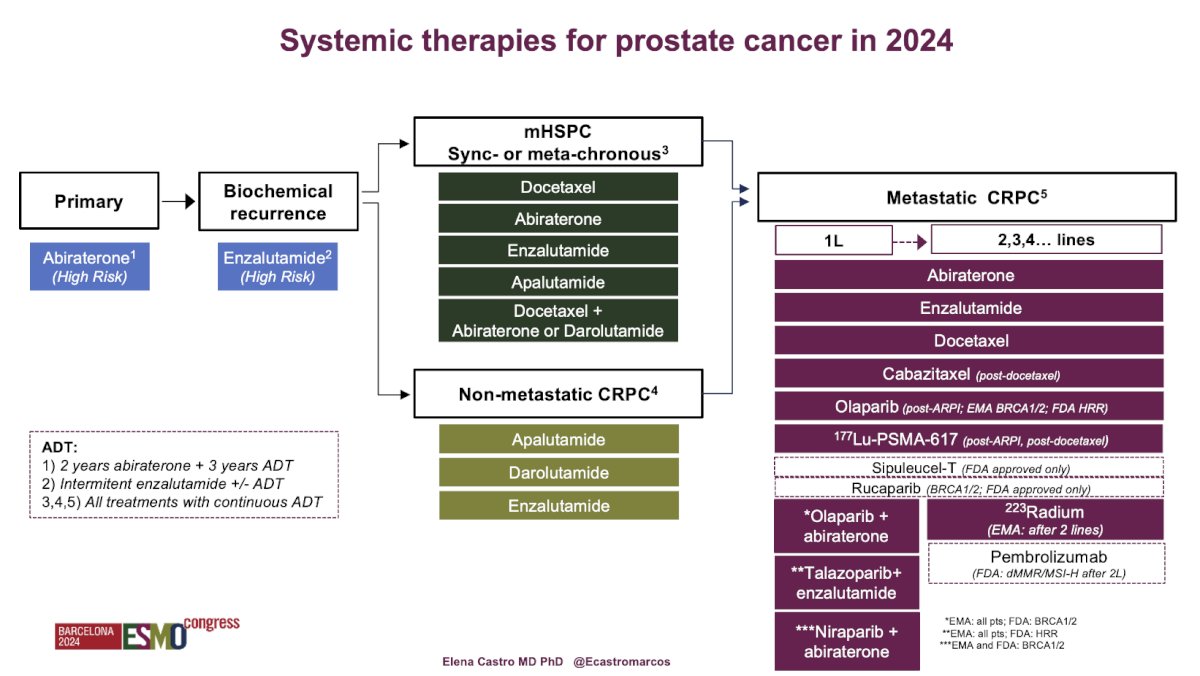
In 2024, the systemic therapies for PCa include:
- Primary setting: Abiraterone for high-risk disease.1
- Biochemical recurrence space: Enzalutamide, with the EMBARK trial demonstrating its efficacy for patients with non-metastatic hormone-sensitive PCa.2
- Non-metastatic castration-resistant prostate cancer (nmCRPC): Approval of three different androgen receptor pathway inhibitors: Darolutamide, Enzalutamide, and Apalutamide.
- Metastatic hormone-sensitive prostate cancer (mHSPC): Inclusion of triplet therapies (Abiraterone + Docetaxel + ADT or Darolutamide + Docetaxel + ADT) and duplet therapies.
- Metastatic castration-resistant prostate cancer (mCRPC): Migration of multiple treatment options to first or second line, including PARP inhibitors combined with ARPIs, Enzalutamide with Ra223, or radioligand therapy with 177Lu-PSMA-617.
Dr. Castro presented a comprehensive summary of all systemic therapies for PCa in 2024, highlighting the evolving and broadening treatment options available to patients as outlined below:
While having multiple treatment options for different stages of PCa is beneficial, it also means that disease progression after ARPIs can occur in various scenarios: after primary treatment, biochemical recurrence, nmCRPC, mHSPC, or mCRPC. Each of these progressions leaves us with more limited options for second/third line therapies. However, in the primary and biochemical recurrence treatment spaces, there is still a limited duration of ARPI±ADT, unlike in mHSPC, nmCRPC, and mCRPC, where patients receive continuous ARPI+ADT until disease progression.
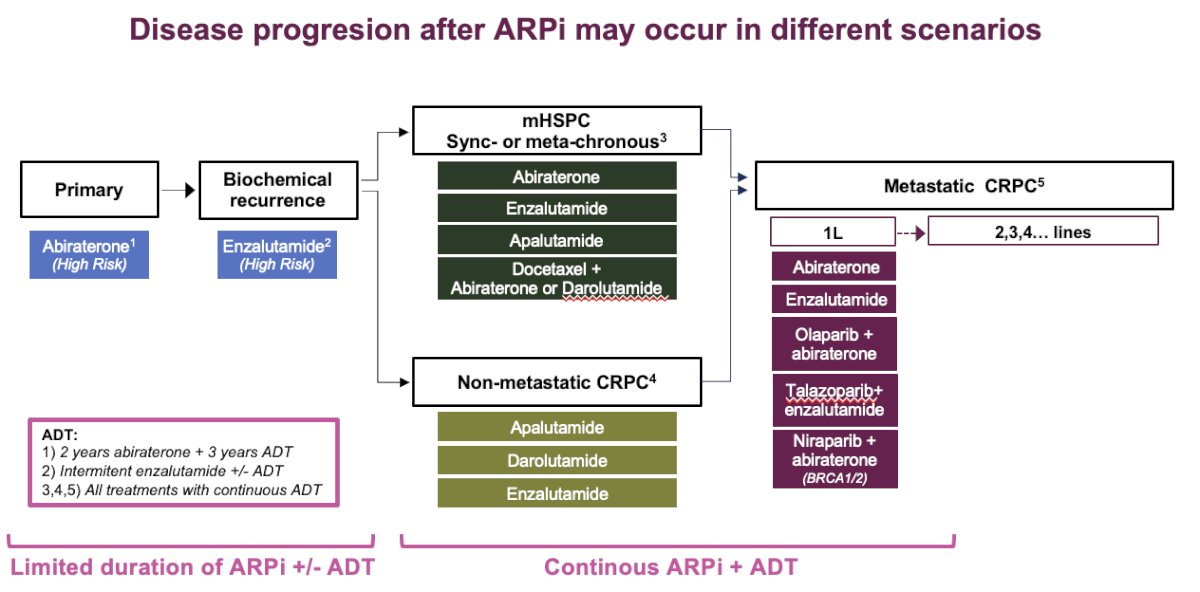
With these introduction, Dr Castro posed the question if cytotoxics are still the next option in PCa? And she discussed after three different scenarios:
- Continuous ARPi + ADT for advanced disease
- Limited duration of ARPi + ADT for localized disease
- Limited duration of ARPi +/- ADT for biochemical recurrence
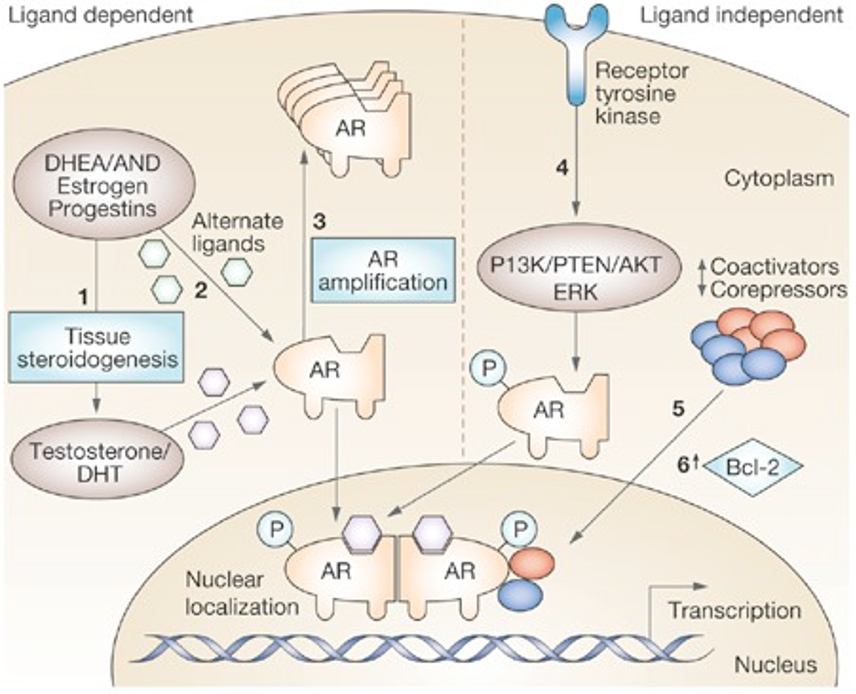
Dr Castro discussed the different mechanisms of resistance to androgen receptor (AR) pathway inhibition. There is a multifaceted resistance landscape involving both AR-dependent and ligand-independent pathways.
In the AR-dependent context, resistance mechanisms include AR amplification and mutations, which enhance receptor activity or enable the receptor to function aberrantly despite therapy. Intratumoral androgen synthesis and enzymatic mutations that increase androgen production further contribute to resistance by providing the tumor with a continuous supply of androgens that can activate the AR even in the presence of inhibitors.3,4
In contrast, AR-independent mechanisms offer alternative routes for resistance. AR splice variants, which lack the ligand-binding domain, can drive cancer progression independently of androgens. Additionally, the interplay between AR and other signaling pathways, such as glucocorticoid receptor (GR) cross-stimulation of AR target genes and alternative oncogenic signals like PTEN loss or neuroendocrine differentiation, complicates treatment by allowing tumors to bypass AR inhibition through these alternative pathways.3,4
Overall, the complexity of resistance mechanisms underscores the necessity for innovative treatment approaches that target both AR-dependent and AR-independent pathways to effectively manage and overcome resistance in PCa. Both the AR-dependent and AR-independent and ligand dependent and ligand-independent pathways are illustrated in the graphic below:
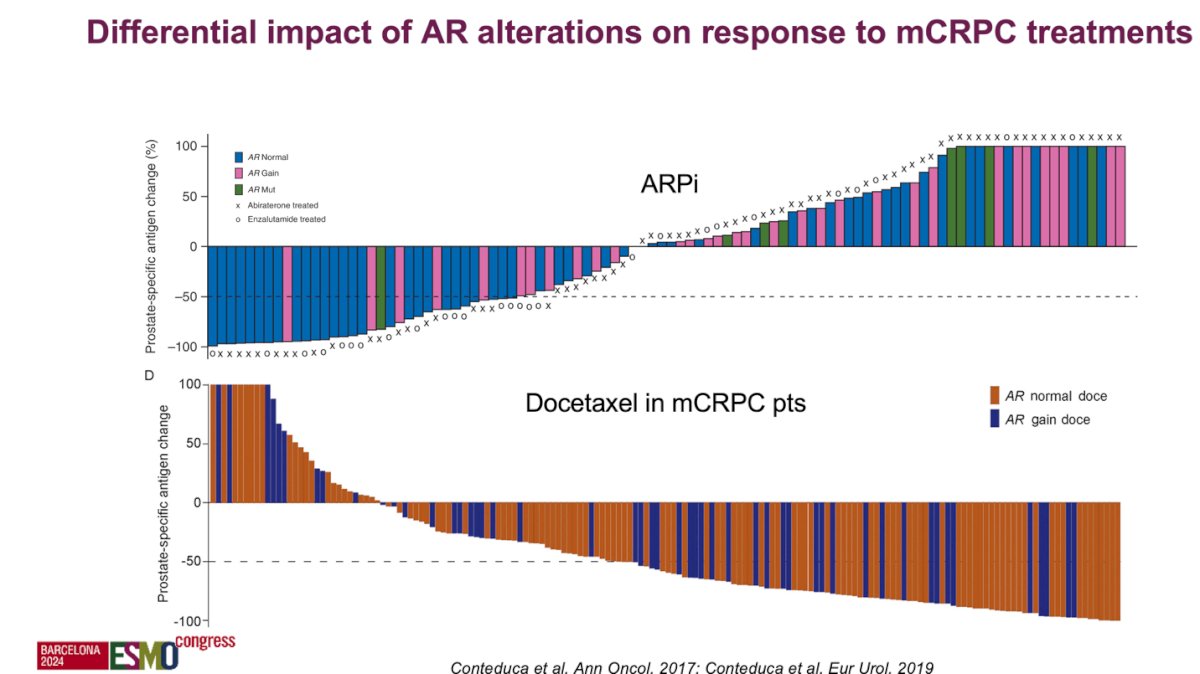
Understanding the mechanisms of resistance to androgen AR pathway and the AR alterations have a differential impact on response to therapy in patients with mCRPC. For example in a multi-institutional study pooling the analysis of AR status, determined by droplet digital polymerase chain reaction (ddPCR), on pretreatment plasma samples of patients with mCRPC, overall survival (OS) was significantly shorter in patients with AR gain, but progression-free survival (PFS) and PSA response were not significantly affected by AR gain. Also, a significant interaction between plasma AR levels and treatment type was observed, specifically, in taxane-naïve patients, abiraterone/enzalutamide was associated with better OS for AR-normal patients, whereas docetaxel showed a trend toward improved outcomes for AR-gained patients as illustrated in the figures below:5
The androgen receptor splice variant-7 (AR-V7) may differentially impact responses to mCRPC treatments, particularly for patients receiving second-line ARPIs and taxanes, and is associated with poor prognosis. A prospective study found that CTC+/AR-V7+ patients had worse oncological outcomes and a higher likelihood of poor prognosis.6 Additionally, another study demonstrated that detecting AR-V7-positive CTCs before therapy could indicate a better survival benefit with taxanes over ARPIs in mCRPC patients, highlighting AR-V7 as a potential treatment-specific biomarker for guiding therapy selection.7 These findings are illustrated in the graphics below:
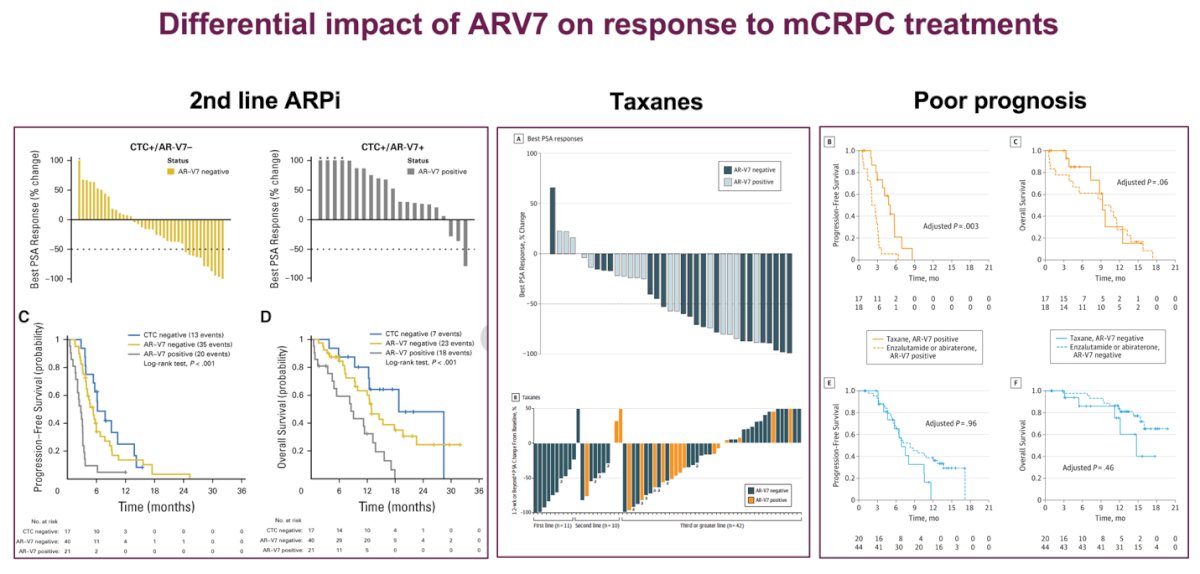
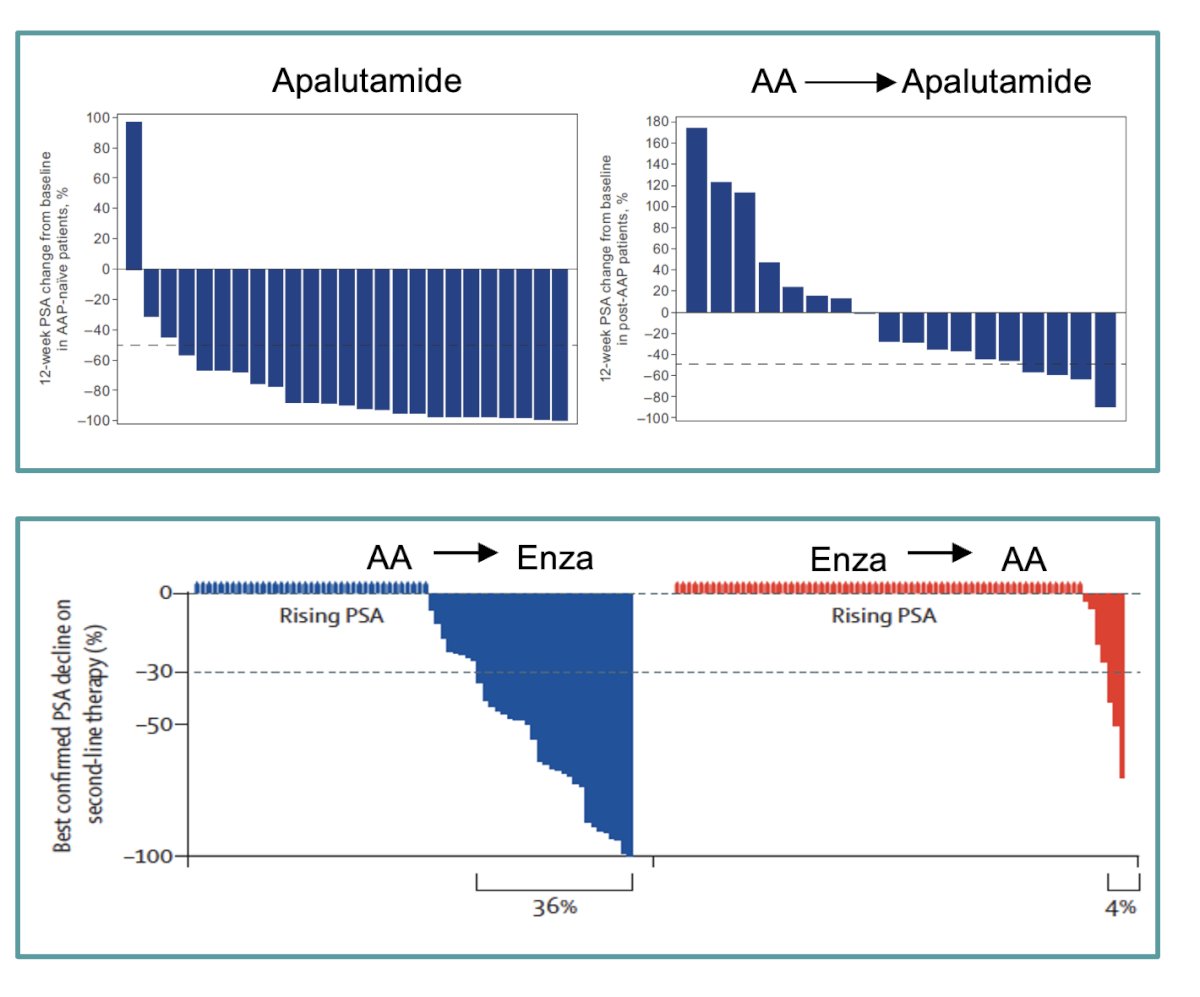
Consistent findings across multiple studies indicate a limited response to second-line ARPIs in men with mCRPC. Dr. Castro emphasized the poor efficacy observed when switching from one ARPI to another, noting a significant difference in PSA response between patients who transitioned from Abiraterone acetate (AA) to Enzalutamide versus those who switched from Enzalutamide to AA. Specifically, only 36% of patients who switched to Enzalutamide showed PSA responses, compared to just 4% for those switching to AA.8 Additionally, apalutamide demonstrated clinical activity in mCRPC patients who had previously received AA and prednisone. Notably, 80% of AA-naïve patients and 43% of post-AA patients remained on apalutamide treatment for 6 months or longer, suggesting that apalutamide can be effective in mCRPC, though response rates vary based on prior treatment history.9 Sequencing matters noted Dr Castro.
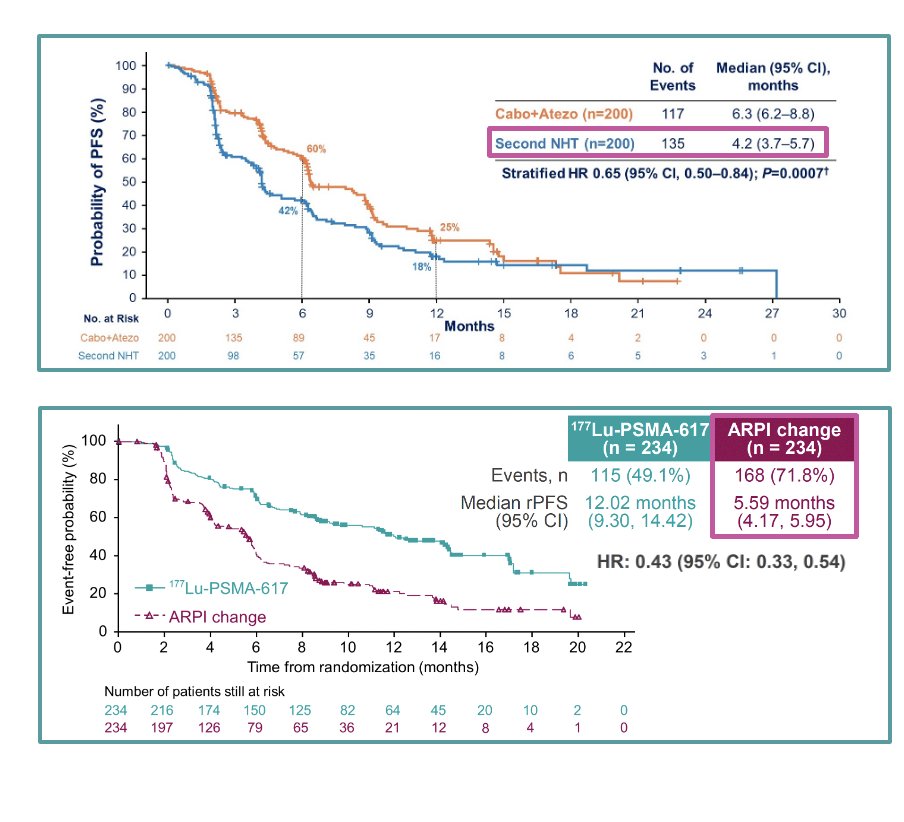
Dr. Castro presented recent findings from the CONTACT-02 Phase 3 study, which compared cabozantinib plus atezolizumab to a second ARPI in mCRPC patients who had progressed on a prior ARPI. The study demonstrated a significant improvement in progression-free survival (PFS) for those treated with cabozantinib plus atezolizumab compared to those receiving a second ARPI. Similarly, the PSMAfore trial, a Phase 3 study involving 177Lu-PSMA-617 in taxane-naive mCRPC patients who progressed after one line of ARPI, showed a clear benefit in radiological PFS (rPFS) for patients treated with the radioligand therapy over a second ARPI. These studies highlight the potential advantages of novel therapeutic approaches or conventional systemic therapy beyond ARPIs in managing mCRPC.
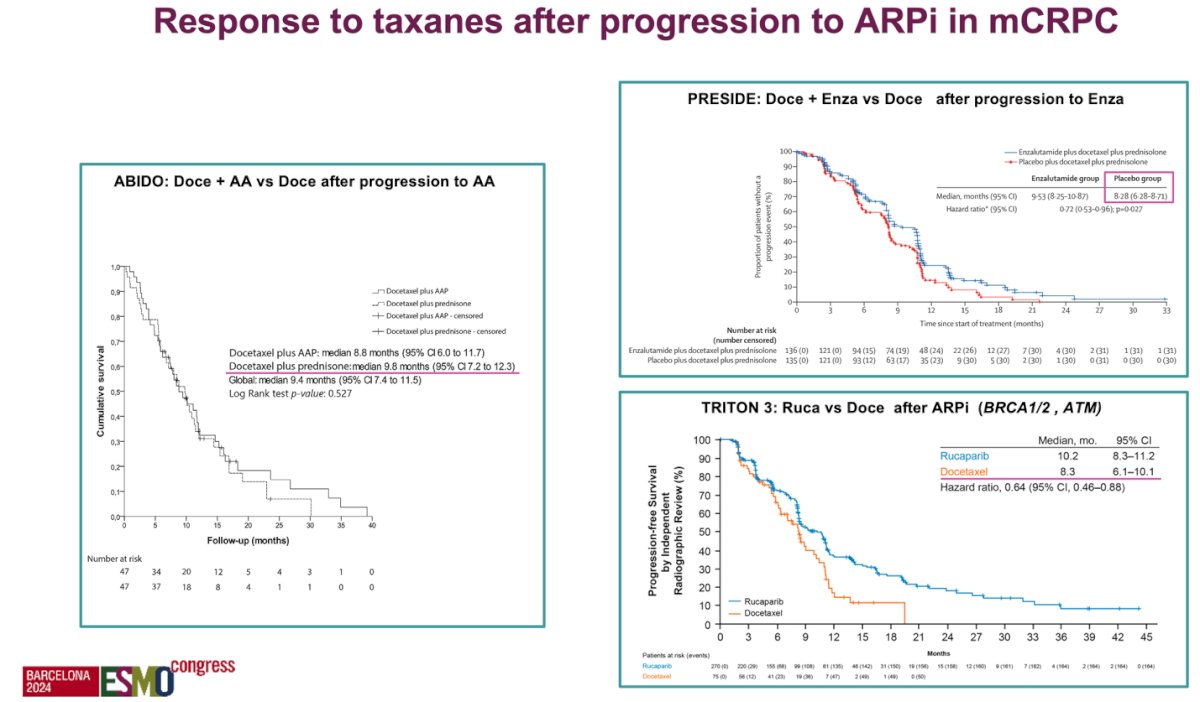
Dr. Castro discussed the efficacy of taxanes following progression on an ARPI in mCRPC patients, citing three key clinical trials. The ABIDO trial compared docetaxel plus abiraterone acetate (AA) to docetaxel alone after progression on AA, while the PRESIDE trial assessed docetaxel plus enzalutamide versus docetaxel after progression on enzalutamide. Both trials consistently found no additional benefit from continuing an ARPI during docetaxel treatment after progression.12,13 In contrast, the TRITON 3 trial evaluated rucaparib versus docetaxel after ARPI progression in patients with homologous recombination repair (HRR) mutations, specifically BRCA1/2 and ATM. This trial showed that rucaparib improved radiological progression-free survival (rPFS) in patients with BRCA1/2 and ATM mutations compared to docetaxel, suggesting a targeted benefit for these specific genetic alterations.14 Duraiton of response appears to be longer with taxanes than with a second ARPI.
The CARD study, a randomized, open-label, multicentric trial, compared cabazitaxel to abiraterone or enzalutamide in mCRPC patients who had previously received docetaxel and an alternative ARPI. Patients were randomized 1:1 to receive either cabazitaxel (25 mg/m² IV every 3 weeks with prednisone and G-CSF) or a second ARPI (abiraterone 1000 mg PO with prednisone or enzalutamide 160 mg PO). Randomization was stratified by ECOG performance status, time to progression on prior ARPI, and ARPI timing relative to docetaxel. The study demonstrated that cabazitaxel was superior to a second ARPI in the third-line setting, showing better outcomes in terms of overall survival (OS) and radiological progression-free survival (rPFS).15
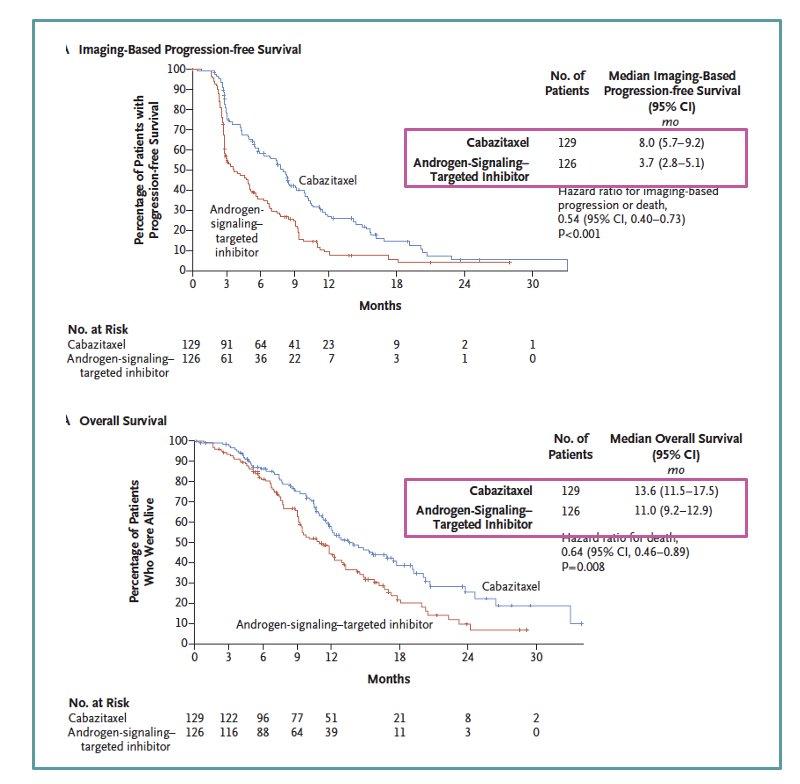
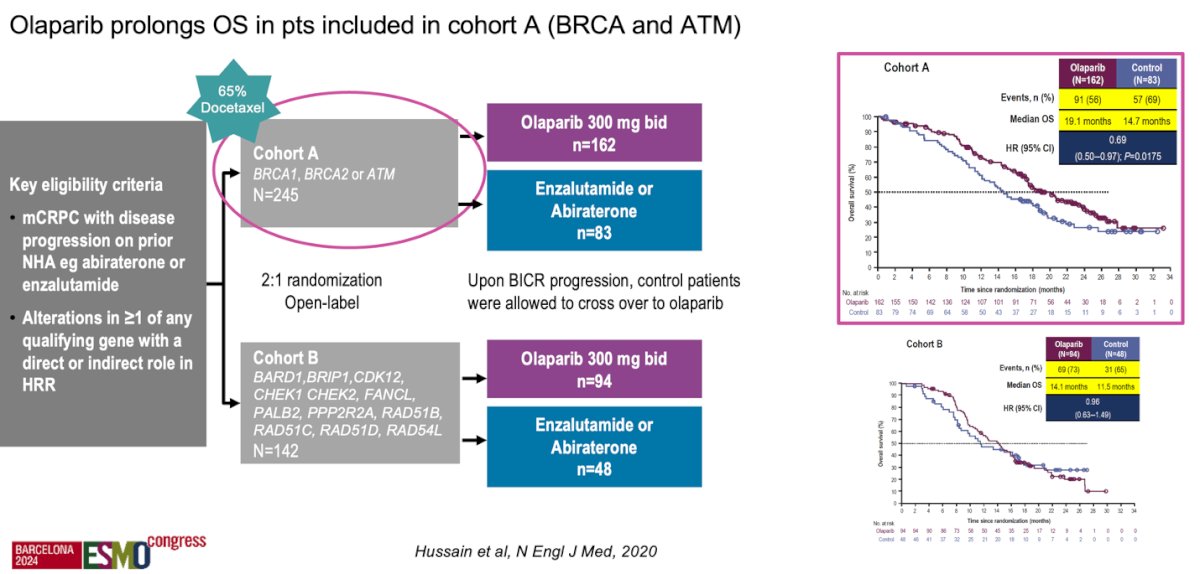
Moreover, the PROfound study, a prospective, biomarker-selected, phase 3 trial involving men with mCRPC who had disease progression while receiving an ARPI and who had a qualifying alteration in prespecified genes (HRRm), randomly assigned participants to receive either the PARP inhibitor olaparib or the physician’s choice of ARPI. One of the criticisms of the study is that up to 65% of patients received docetaxel and this leaves us with the question of whether the PARPi should have been given before or after docetaxel. The PARPi was superior to ARPI switch regardless of whether patients had received docetaxel. Olaparib prolonged OS in patients included in cohort A (BRCA 1/2 and ATM mutations), strengthening the need for another systemic therapy after progression on ARPI in mCRPC.16
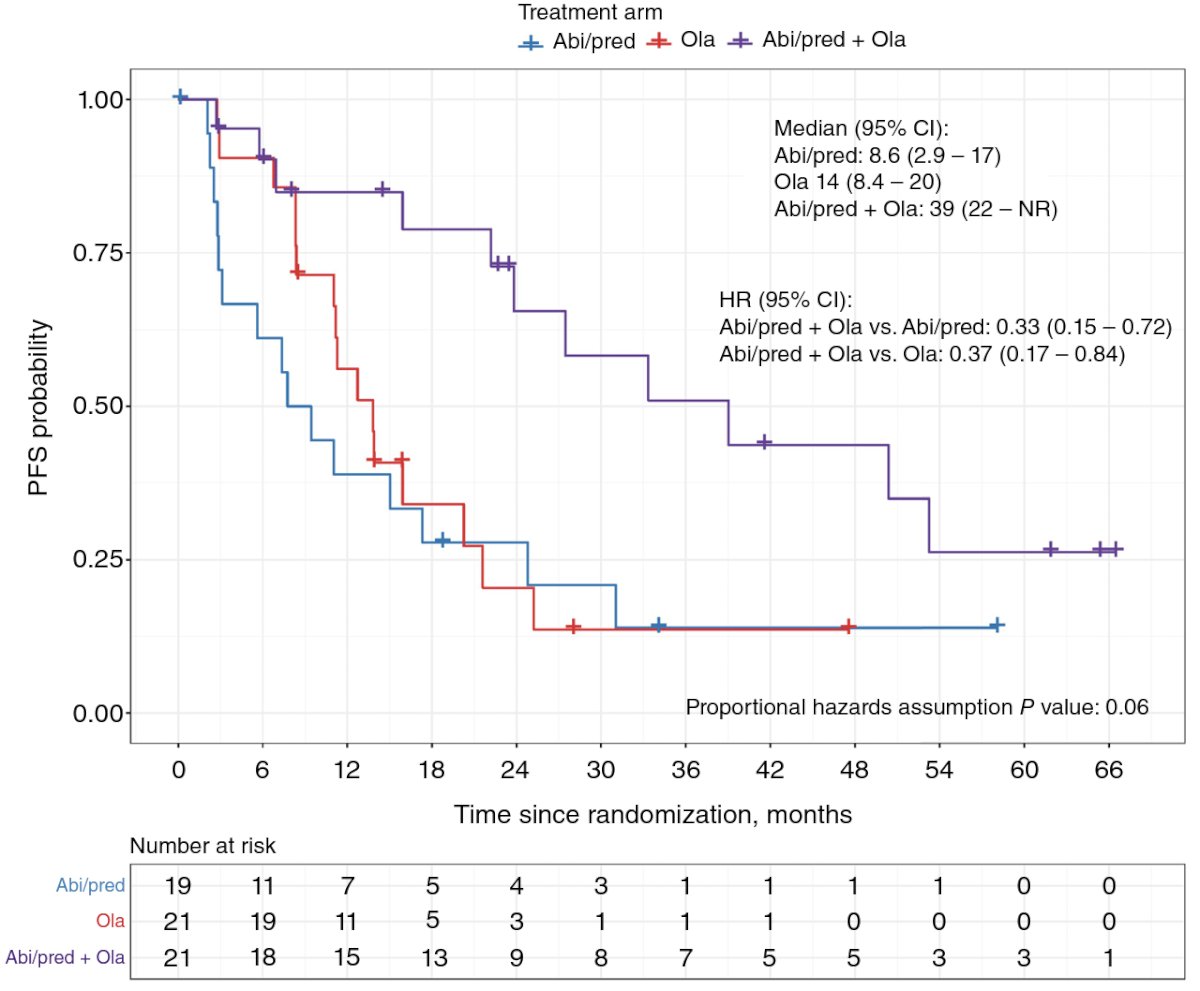
She moved on to discuss the BRCAAway phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with mCRPC bearing HRRm with no prior exposure to PARPi, ARPIs, or chemotherapy. Patients with inactivating BRCA1/2 and/or ATM alterations were randomized 1:1:1 to Arm I (abiraterone 1000 mg qd + prednisone 5 mg bid), Arm II (olaparib 300 mg bid), or Arm III (olaparib + abiraterone/prednisone). This study showed that in mCRPC patients with BRCA1/2 or ATM alterations, abiraterone/prednisone + olaparib was well tolerated and resulted in a longer PFS compared to either agent alone or sequentially.18 In clinical practice it is quite common that one patient who becomes mCRPC has received prior ARPI, leaving us with the question of sequencing to a second ARPI after progression.
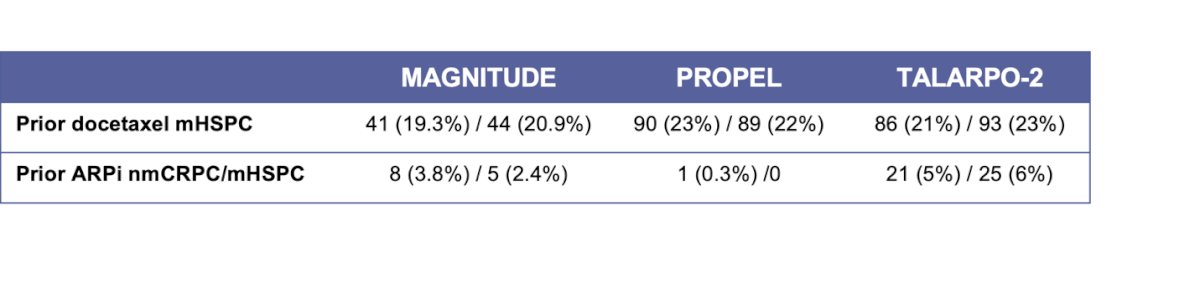
Dr. Castro highlighted that in BRCA-mutated patients, PARPi + ARPI may be better than PARPi alone in the first-line of mCRPC. However, she posed the question of whether this is true even after progression on a prior ARPI. She discussed that the approval by the FDA and the EMA for Olaparib + AA, Talazoparib + Enzalutamide, and Niraparib + AA in BRCA1/2-mutated patients or in all comers has presented a new clinical dilemma. We don’t know if the patients who had received prior ARPI in the mHSPC setting or in the nmCRPC setting would respond to the combination of PARPi and ARPI. She showed that in the three pivotal clinical trials, prior ARPI exposure in nmCRPC/mHSPC was low, ranging from 0.3% to 6%, as illustrated below, leaving this question unanswered.
Moreover, the PSMAfore study comparing 177Lu-PSMA-617 to a second ARPI showed a significant benefit in rPFS for patients treated with the radioligand therapy. However, regarding OS, the third interim analysis demonstrated a hazard ratio of 0.98 (95% CI: 0.75–1.28), with a data maturity of 44.4–47.9%. The median overall survival was 23.7 months and 23.9 months in the two arms, respectively. Notably, 77.5% of eligible patients in the ARPI change group crossed over to 177Lu-PSMA-617 treatment.11
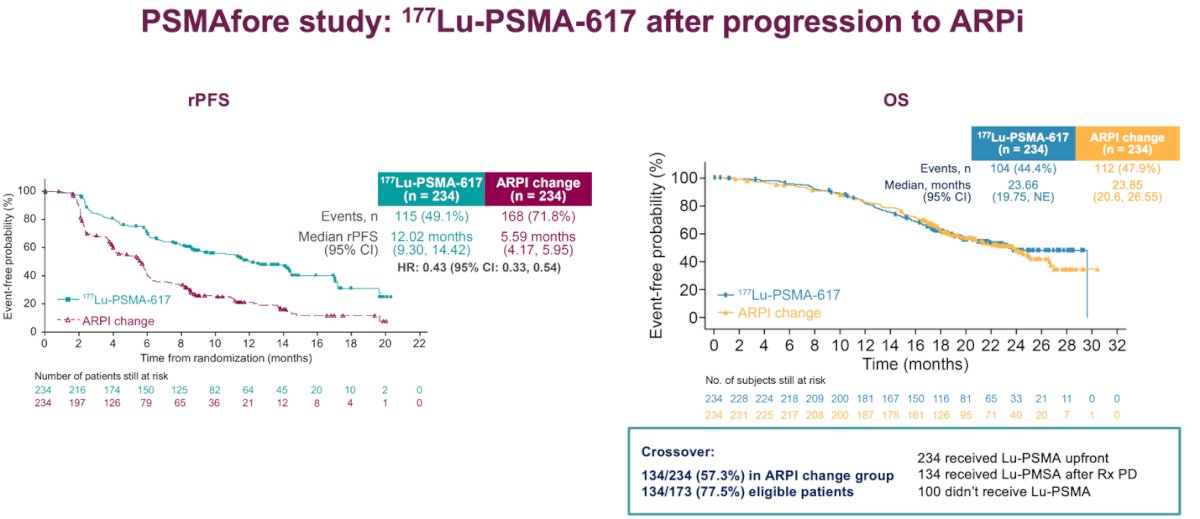
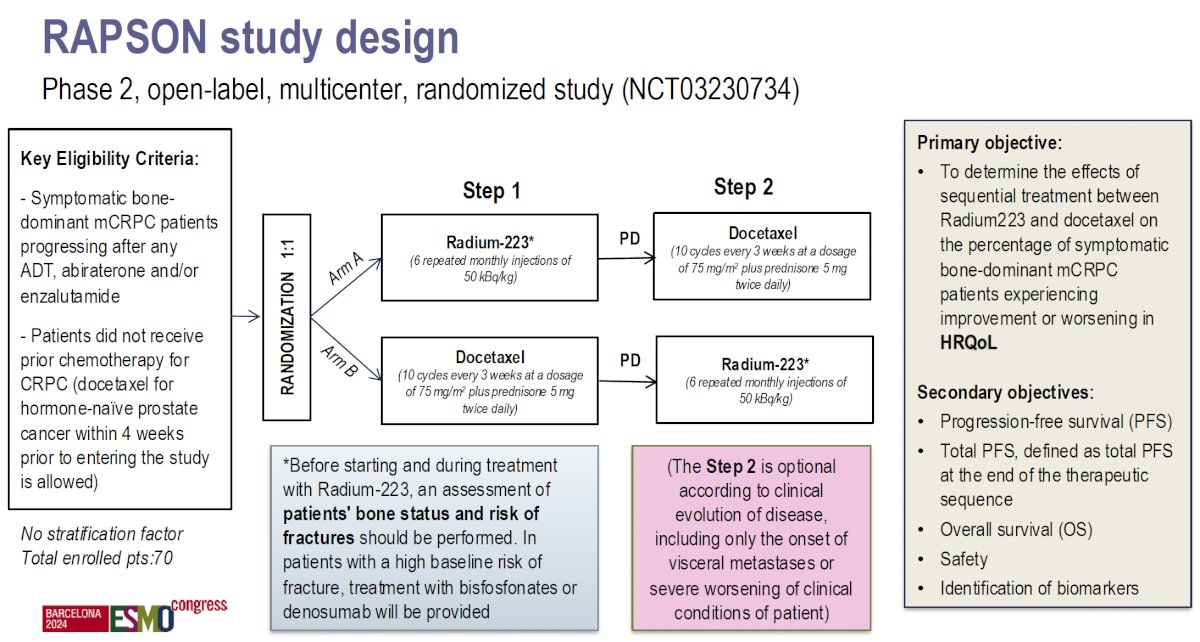
Lastly, she mentioned a late-breaking abstract that will be presented at ESMO 2024: the RAPSON study, which will be presented on Monday. This study is an open-label, multicentre, randomised trial of Radium-223 followed by docetaxel versus docetaxel followed by Radium-223, aiming to determine the optimal sequencing for these drugs and may help to answer our questions. The study design is shown below:
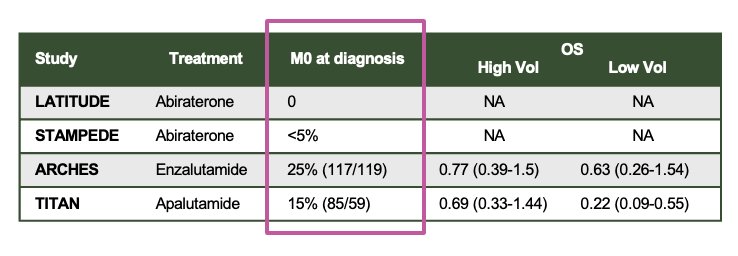
Dr. Castro discussed the scenario of patients treated with curative intent who received limited-duration ARPI (2 years) and ADT. She reviewed the data for patients in the mHSPC setting. Notably, most of the patients included in the pivotal trials had synchronous mHSPC. In LATITUDE, 0% had M0 disease at diagnosis, while <5% in STAMPEDE, 25% in ARCHES, and 15% in TITAN had M0 disease at diagnosis, as shown below:
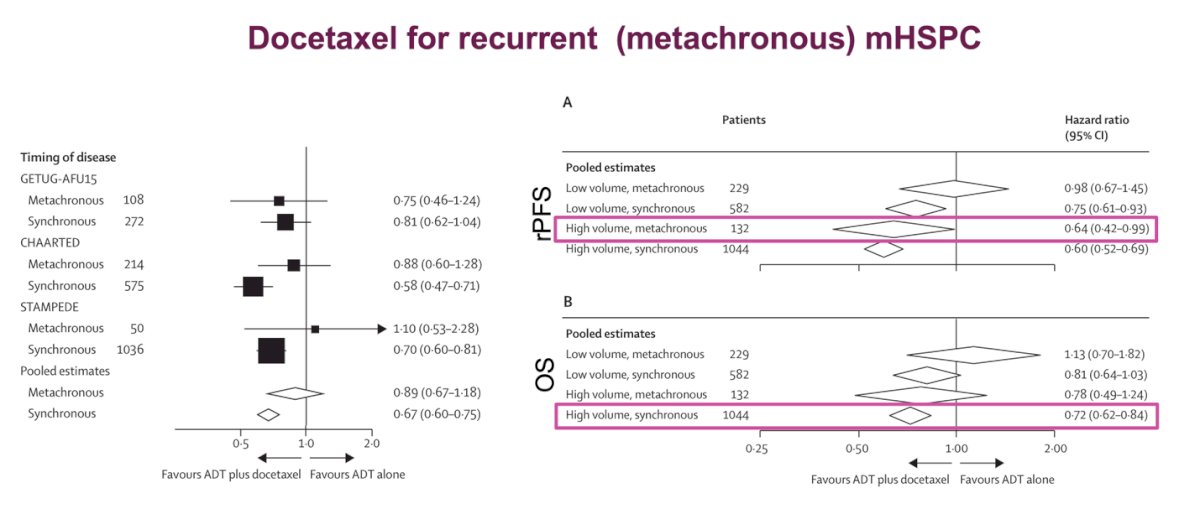
This raises the question of whether ARPI would be the best treatment strategy for patients who received limited-duration ARPI + ADT for localized disease and then progressed to mHSPC. The role of docetaxel for recurrent (metachronous) mHSPC was studied recently in a systematic review and meta-analysis of individual participant data from three clinical trials (GETUG-AFU15, CHAARTED, and STAMPEDE). This study showed that the addition of docetaxel to hormone therapy is best suited for patients with a high volume of disease, regardless of whether the mHSPC was metachronous or synchronous, in terms of rPFS. However, there is no evidence of a meaningful benefit for patients with metachronous, low-volume disease in either OS or rPFS. In terms of OS, the only group that showed a benefit from adding docetaxel to ADT was the high-volume synchronous group, which again suggests that metachronous patients, regardless of volume, may not be ideal candidates for docetaxel.19
Dr. Castro discussed whether triplet therapy might be better suited for patients with recurrent (metachronous) mHSPC. She reviewed data from ARASENS, a phase 3 trial, where patients with mHSPC were randomized 1:1 to receive darolutamide or a matching placebo, both in combination with ADT and docetaxel. The study showed a significant improvement in OS with triplet therapy. However, the representation of patients with metachronous disease was low; in the high-volume group of patients treated with darolutamide and placebo, only 11.7% and 11.6%, respectively, had metachronous mHSPC.20
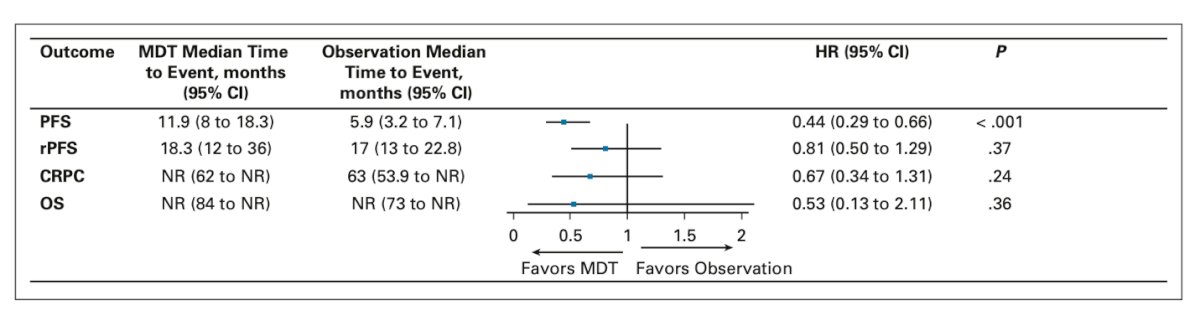
Lastly, she discussed the role of SBRT for oligorecurrent disease (low-volume metachronous mHSPC). She presented the long-term outcome analysis from the STOMP and ORIOLE trials, which showed a significant benefit in progression-free survival (PFS). However, there was no benefit of SBRT in terms of radiographic progression-free survival (rPFS), time to castration-resistant prostate cancer (CRPC), or overall survival (OS).21
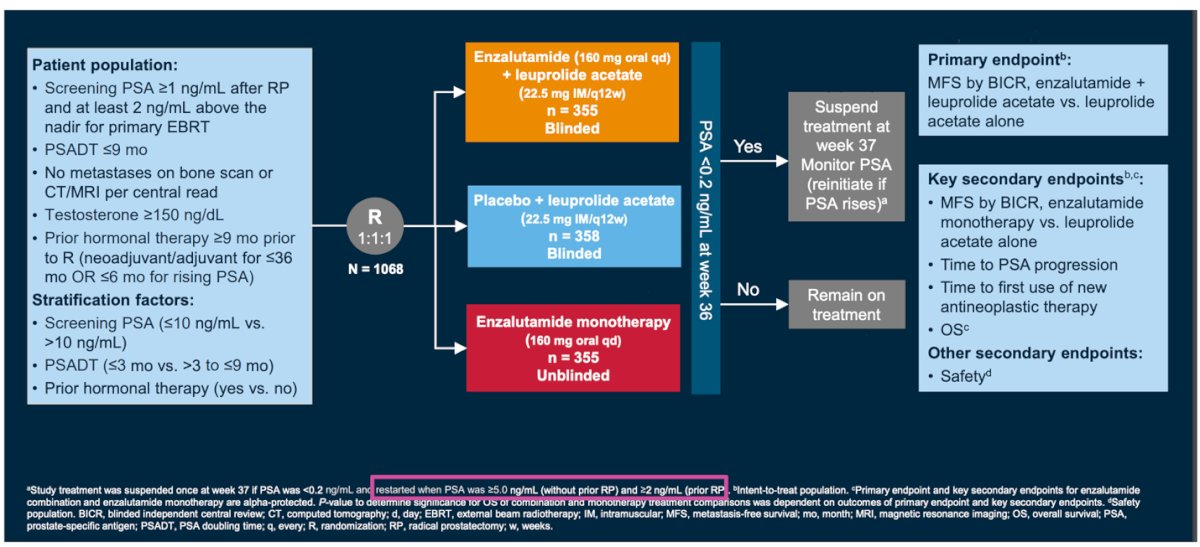
The Embark study, a phase 3 study of patients with nonmetastatic hormone-sensitive prostate cancer with high-risk BCR (PSA doubling time ≤9 months, screening PSA ≥2 ng/mL above nadir post radiotherapy or ≥1 ng/mL post radical prostatectomy) were randomized (1:1:1) to enzalutamide + leuprolide acetate, enzalutamide alone, and placebo + leuprolide acetate. Study design below:
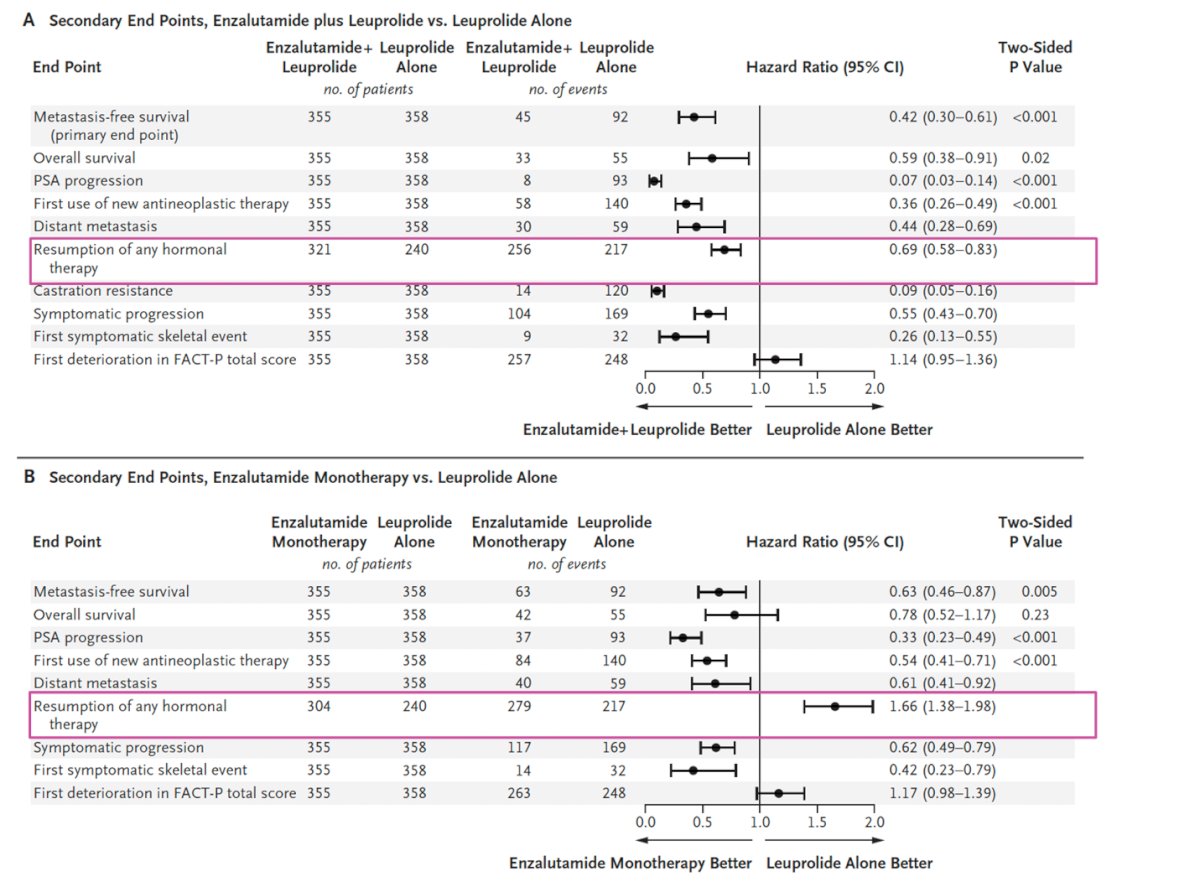
Dr. Castro highlighted that study treatment was suspended at week 37 if PSA levels were ≤0.2 ng/mL but was quickly restarted when PSA levels reached ≥5.0 ng/mL. Regarding metastasis-free survival, enzalutamide plus leuprolide was superior to leuprolide alone (hazard ratio for metastasis or death, 0.42; 95% CI, 0.30 to 0.61; P < 0.001). Enzalutamide monotherapy also showed superiority compared to leuprolide alone. Notably, the enzalutamide plus leuprolide group experienced a significantly prolonged time before resuming any hormonal therapy after treatment (HR 0.69; 95% CI, 0.58-0.83), whereas this effect was not observed in the enzalutamide monotherapy arm, meaning that all these patients would eventually need treatment on progression.
Dr Castro wrapped up her presentation with the following messages:
- Cytotoxics are not always the next treatment option.
- In patients who had received continuous ARPI + ADT for advanced disease
-There is a limited response to second ARPI
- Taxanes are active after progression to ARPI
- For BRCA patients, PARPi seem superior to taxanes
- 177Lu-PSMA-617 yet to be approved after ARPI
- 223Radium or taxanes first, still to be determined, we don’t have this answer yet.
- In patients who had limited duration of ARPi + ADT for localized disease
- There are no available treatment options assessed in patients treated in this scenario so we can only extrapolate the data we have from patients with recurrent disease.
- Only a small number of patients with recurrent mHSPC included in Randomized controlled trials
- SBRT for oligorecurrent mHSPC still under study but may become an option. We need more mature data
- In patients with limited duration of ARPi +/- ADT for biochemical recurrence the resumption of hormonal treatment (ARPi+/- ADT) seems the preferred treatment of choice according to data from EMBARK.
Presented by: Elena Castro, MD, PhD, Medical Oncologist. Hospital Universitario 12 de Octubre, Madrid, Spain.
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, Gillessen S, Cook A, Brawley C, Amos CL, Atako N, Pugh C, Buckner M, Chowdhury S, Malik Z, Russell JM, Gilson C, Rush H, Bowen J, Lydon A, Pedley I, O'Sullivan JM, Birtle A, Gale J, Srihari N, Thomas C, Tanguay J, Wagstaff J, Das P, Gray E, Alzoueb M, Parikh O, Robinson A, Syndikus I, Wylie J, Zarkar A, Thalmann G, de Bono JS, Dearnaley DP, Mason MD, Gilbert D, Langley RE, Millman R, Matheson D, Sydes MR, Brown LC, Parmar MKB, James ND; Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022 Jan 29;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5. Epub 2021 Dec 23. PMID: 34953525; PMCID: PMC8811484.
- LBA02-09 EMBARK: A Phase 3 Randomized Study of Enzalutamide or Placebo Plus Leuprolide Acetate and Enzalutamide Monotherapy in High-risk Biochemically Recurrent Prostate Cancer. J Urol. 2023 Jul;210(1):224-226. doi: 10.1097/JU.0000000000003518. Epub 2023 May 2. PMID: 37119051.
- Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015 Mar;67(3):470-9. doi: 10.1016/j.eururo.2014.09.049. Epub 2014 Oct 8. PMID: 25306226; PMCID: PMC5301306.
- Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009 Feb;6(2):76-85. doi: 10.1038/ncpuro1296. Erratum in: Nat Clin Pract Urol. 2009 Mar;6(3):173. PMID: 19198621; PMCID: PMC2981403.
- Conteduca V, Jayaram A, Romero-Laorden N, Wetterskog D, Salvi S, Gurioli G, Scarpi E, Castro E, Marin-Aguilera M, Lolli C, Schepisi G, Maugeri A, Wingate A, Farolfi A, Casadio V, Medina A, Puente J, Vidal MJM, Morales-Barrera R, Villa-Guzmán JC, Hernando S, Rodriguez-Vida A, González-Del-Alba A, Mellado B, Gonzalez-Billalabeitia E, Olmos D, Attard G, De Giorgi U. Plasma Androgen Receptor and Docetaxel for Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2019 Mar;75(3):368-373. doi: 10.1016/j.eururo.2018.09.049. Epub 2018 Oct 26. PMID: 30773204; PMCID: PMC6377278.
- Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR, Pienta KJ, Paller CJ, Carducci MA, Eisenberger MA, Luo J. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J Clin Oncol. 2017 Jul 1;35(19):2149-2156. doi: 10.1200/JCO.2016.70.1961. Epub 2017 Apr 6. PMID: 28384066; PMCID: PMC5493048.
- Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, McLaughlin B, Wahl J, Greene SB, Heller G, Marrinucci D, Fleisher M, Dittamore R. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016 Nov 1;2(11):1441-1449. doi: 10.1001/jamaoncol.2016.1828. Erratum in: JAMA Oncol. 2016 Nov 1;2(11):1511. doi: 10.1001/jamaoncol.2016.4680. PMID: 27262168; PMCID: PMC5206761.
- Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, Zulfiqar M, Sunderland K, Azad AA, Kollmannsberger CK, Eigl BJ, Noonan K, Wadhwa D, Attwell A, Keith B, Ellard SL, Le L, Gleave ME, Wyatt AW, Chi KN. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019 Dec;20(12):1730-1739. doi: 10.1016/S1470-2045(19)30688-6. Epub 2019 Nov 11. PMID: 31727538.
- Rathkopf DE, Antonarakis ES, Shore ND, Tutrone RF, Alumkal JJ, Ryan CJ, Saleh M, Hauke RJ, Bandekar R, Maneval EC, de Boer CJ, Yu MK, Scher HI. Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone. Clin Cancer Res. 2017 Jul 15;23(14):3544-3551. doi: 10.1158/1078-0432.CCR-16-2509. Epub 2017 Feb 17. PMID: 28213364; PMCID: PMC5543693.
- Agarwal N, Azad A, Carles J, Chowdhury S, McGregor B, Merseburger AS, Oudard S, Saad F, Soares A, Benzaghou F, Kerloeguen Y, Kimura A, Mohamed N, Panneerselvam A, Wang F, Pal S. A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol. 2022 Mar;18(10):1185-1198. doi: 10.2217/fon-2021-1096. Epub 2022 Jan 17. PMID: 35034502.
- Sartor, O. et al. LBA13 Phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Annals of Oncology, Volume 34, S1324 - S1325
- Climent MA, Font A, Durán I, Puente J, José Méndez-Vidal M, Sáez MI, Santander Lobera C, Ángel Arranz Arija J, González-Del-Alba A, Sánchez-Hernandez A, Juan Fita MJ, Esteban E, Alonso-Gordoa T, Mellado Gonzalez B, Maroto P, Lázaro-Quintela M, Cassinello-Espinosa J, Pérez-Valderrama B, Garcias C, Castellano D. A phase II randomised trial of abiraterone acetate plus prednisone in combination with docetaxel or docetaxel plus prednisone after disease progression to abiraterone acetate plus prednisone in patients with metastatic castration-resistant prostate cancer: The ABIDO-SOGUG trial. Eur J Cancer. 2022 Nov;175:110-119. doi: 10.1016/j.ejca.2022.08.002. Epub 2022 Sep 11. PMID: 36099670.
- Merseburger AS, Attard G, Åström L, Matveev VB, Bracarda S, Esen A, Feyerabend S, Senkus E, López-Brea Piqueras M, Boysen G, Gourgioti G, Martins K, Chowdhury S. Continuous enzalutamide after progression of metastatic castration-resistant prostate cancer treated with docetaxel (PRESIDE): an international, randomised, phase 3b study. Lancet Oncol. 2022 Nov;23(11):1398-1408. doi: 10.1016/S1470-2045(22)00560-5. Epub 2022 Oct 18. PMID: 36265504.
- Fizazi K, Piulats JM, Reaume MN, Ostler P, McDermott R, Gingerich JR, Pintus E, Sridhar SS, Bambury RM, Emmenegger U, Lindberg H, Morris D, Nolè F, Staffurth J, Redfern C, Sáez MI, Abida W, Daugaard G, Heidenreich A, Krieger L, Sautois B, Loehr A, Despain D, Heyes CA, Watkins SP, Chowdhury S, Ryan CJ, Bryce AH; TRITON3 Investigators. Rucaparib or Physician's Choice in Metastatic Prostate Cancer. N Engl J Med. 2023 Feb 23;388(8):719-732. doi: 10.1056/NEJMoa2214676. Epub 2023 Feb 16. PMID: 36795891; PMCID: PMC10064172.
- de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, Kramer G, Eymard JC, Bamias A, Carles J, Iacovelli R, Melichar B, Sverrisdóttir Á, Theodore C, Feyerabend S, Helissey C, Ozatilgan A, Geffriaud-Ricouard C, Castellano D; CARD Investigators. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019 Dec 26;381(26):2506-2518. doi: 10.1056/NEJMoa1911206. Epub 2019 Sep 30. PMID: 31566937.
- de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 May 28;382(22):2091-2102. doi: 10.1056/NEJMoa1911440. Epub 2020 Apr 28. PMID: 32343890.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, Procopio G, de Menezes J, Girotto G, Arslan C, Mehra N, Parnis F, Brown E, Schlürmann F, Joung JY, Sugimoto M, Virizuela JA, Emmenegger U, Navratil J, Buchschacher GL, Poehlein C, Harrington EA, Desai C, Kang J, Saad F. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022 Sep;1(9):EVIDoa2200043. doi: 10.1056/EVIDoa2200043. Epub 2022 Jun 3. PMID: 38319800.
- Maha H. A. Hussain et al. BRCAAway: A randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) bearing homologous recombination-repair mutations (HRRm).. JCO 42, 19-19(2024).
- Vale CL, Fisher DJ, Godolphin PJ, Rydzewska LH, Boher JM, Burdett S, Chen YH, Clarke NW, Fizazi K, Gravis G, James ND, Liu G, Matheson D, Murphy L, Oldroyd RE, Parmar MKB, Rogozinska E, Sfumato P, Sweeney CJ, Sydes MR, Tombal B, White IR, Tierney JF; STOPCAP Collaboration. Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials. Lancet Oncol. 2023 Jul;24(7):783-797. doi: 10.1016/S1470-2045(23)00230-9. PMID: 37414011; PMCID: PMC7616350.
- Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B, Montesa-Pino Á, Ye D, Parnis F, Cruz F, Tammela TLJ, Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H, Thiele S, Li R, Kuss I, Tombal B; ARASENS Trial Investigators. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142. doi: 10.1056/NEJMoa2119115. Epub 2022 Feb 17. PMID: 35179323; PMCID: PMC9844551.
- Deek MP, Van der Eecken K, Sutera P, Deek RA, Fonteyne V, Mendes AA, Decaestecker K, Kiess AP, Lumen N, Phillips R, De Bruycker A, Mishra M, Rana Z, Molitoris J, Lambert B, Delrue L, Wang H, Lowe K, Verbeke S, Van Dorpe J, Bultijnck R, Villeirs G, De Man K, Ameye F, Song DY, DeWeese T, Paller CJ, Feng FY, Wyatt A, Pienta KJ, Diehn M, Bentzen SM, Joniau S, Vanhaverbeke F, De Meerleer G, Antonarakis ES, Lotan TL, Berlin A, Siva S, Ost P, Tran PT. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022 Oct 10;40(29):3377-3382. doi: 10.1200/JCO.22.00644. Epub 2022 Aug 24. PMID: 36001857; PMCID: PMC10166371.
- Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, Haas GP, Kim CS, Ramirez-Backhaus M, Rannikko A, Tarazi J, Sridharan S, Sugg J, Tang Y, Tutrone RF Jr, Venugopal B, Villers A, Woo HH, Zohren F, Shore ND. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023 Oct 19;389(16):1453-1465. doi: 10.1056/NEJMoa2303974. PMID: 37851874.


