(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the session Therapeutic options beyond AR pathway inhibitors: What do we choose next? Dr. Silke Gillessen discussed the role of theranostics after ARPIs and questioned if these will become the next standard of care.
Dr. Gillessen began her presentation by emphasizing that theranostics have already become the standard of care in prostate cancer. She noted that theranostics are endorsed by all clinical practice guidelines. She discussed the updated prostate cancer treatment recommendations from the ESMO clinical practice guideline, which considers treatment intensification and the use of novel systemic agents. These guidelines recommend 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer (mCRPC) following treatment with novel androgen receptor axis inhibitors (ARPIs) and taxanes.1
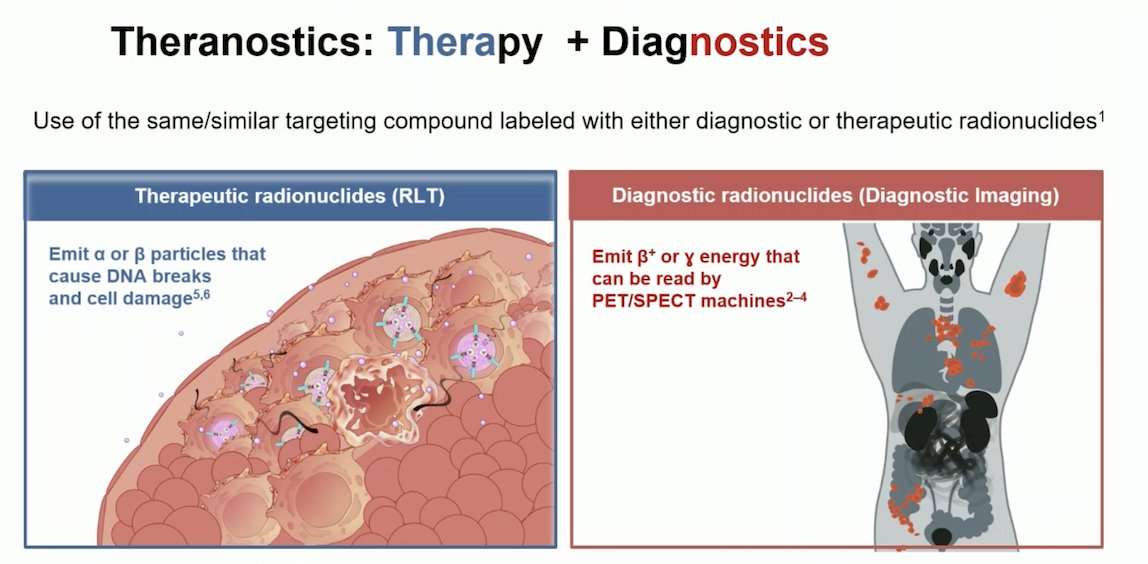
Theranostics, derived from the words "therapy" and "diagnostics," involve the use of the same or similar targeting compounds labeled with either diagnostic or therapeutic radionuclides. The principle behind theranostics is to utilize these compounds for both imaging and treatment purposes. Diagnostic radionuclides emit β+ or γ energy, which can be detected by positron emission tomography (PET) or single-photon emission computed tomography (SPECT) machines, allowing for precise imaging of cancerous tissues. Therapeutic radionuclides, more commonly known as radioligand therapy (RLT), emit α or β particles that cause DNA breaks and ultimately lead to cell damage and death. This dual approach enables personalized treatment plans that can effectively target and manage prostate cancer cells.
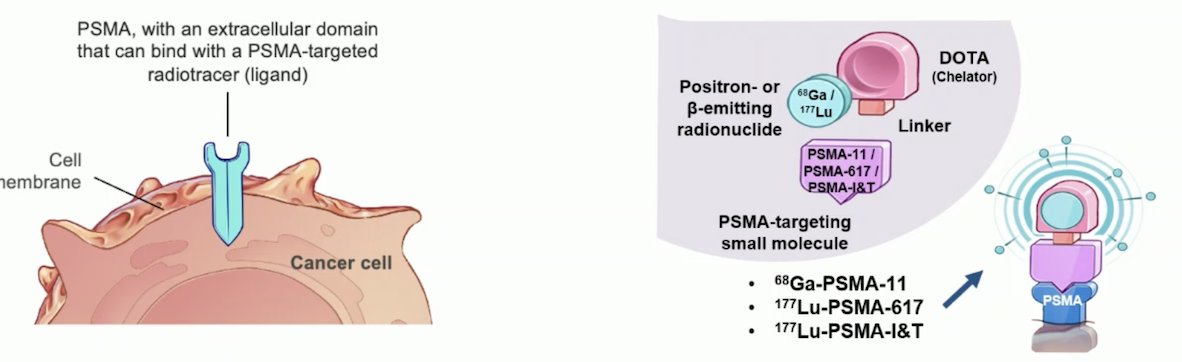
Prostate-specific membrane antigen (PSMA) is highly expressed on the surface of prostate cancer cells. Although it is also present in the kidneys (proximal renal tubules), salivary and lacrimal glands, and proximal small intestine, its limited expression outside of the prostate makes it an ideal target for prostate cancer treatment. This specificity allows theranostics to effectively target prostate cancer cells, minimizing damage to healthy tissues. Various radionuclides like 68Ga-PSMA-11, 177Lu-PSMA-617, and 177Lu-PSMA-I&T can emit positron or β+ energy, precisely targeting PSMA-expressing prostate cancer cells, as illustrated in the graphic below.
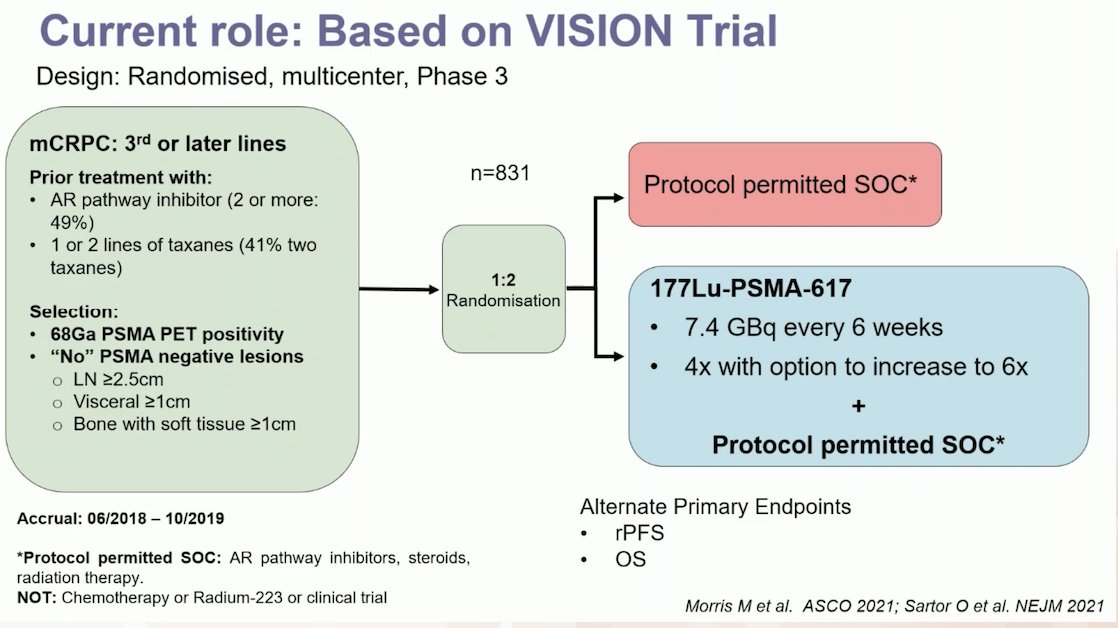
So, if theranostics have become the standard of care for prostate cancer (mCRPC), what is their current role in treating our patients? Dr. Gillessen discussed the VISION trial (NCT03511664), an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with ARPIs (abiraterone, enzalutamide) and one or two prior lines of taxane chemotherapy.
Importantly, patients had to have PSMA-positive disease based on a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver, with no PSMA-negative metastatic lesions. Patients in the Lu-PSMA treatment arm received 4 cycles of the drug, with an option to extend to 6 cycles if a disease response was seen but there was no complete response. The other arm received the standard of care, which included ARPIs, steroids, or radiation therapy.2 The study design and endpoints are illustrated below:
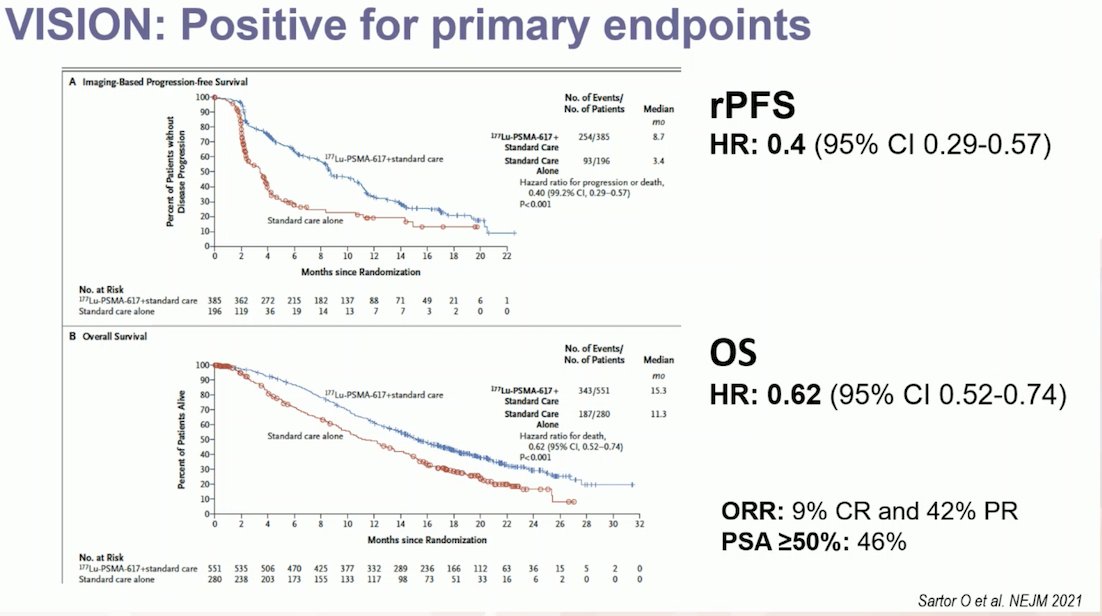
The VISION trial assessed 1,179 patients for eligibility, of which 831 underwent randomization. Notably, 12% of patients were excluded based on 68Ga-PSMA-11 PET results. The trial demonstrated that Lu-PSMA significantly prolonged overall survival (OS) from a median of 11.3 months to 15.3 months, with a hazard ratio of 0.62 (p < 0.001) for death. This OS benefit was consistent across all 831 patients, and among the 581 patients analyzed for radiographic progression-free survival (rPFS), the hazard ratio was 0.4 (95% CI 0.29-0.57). Impressively, 9% of patients achieved a complete response (CR), and 42% achieved a partial response (PR) in the third-line setting of mCRPC.
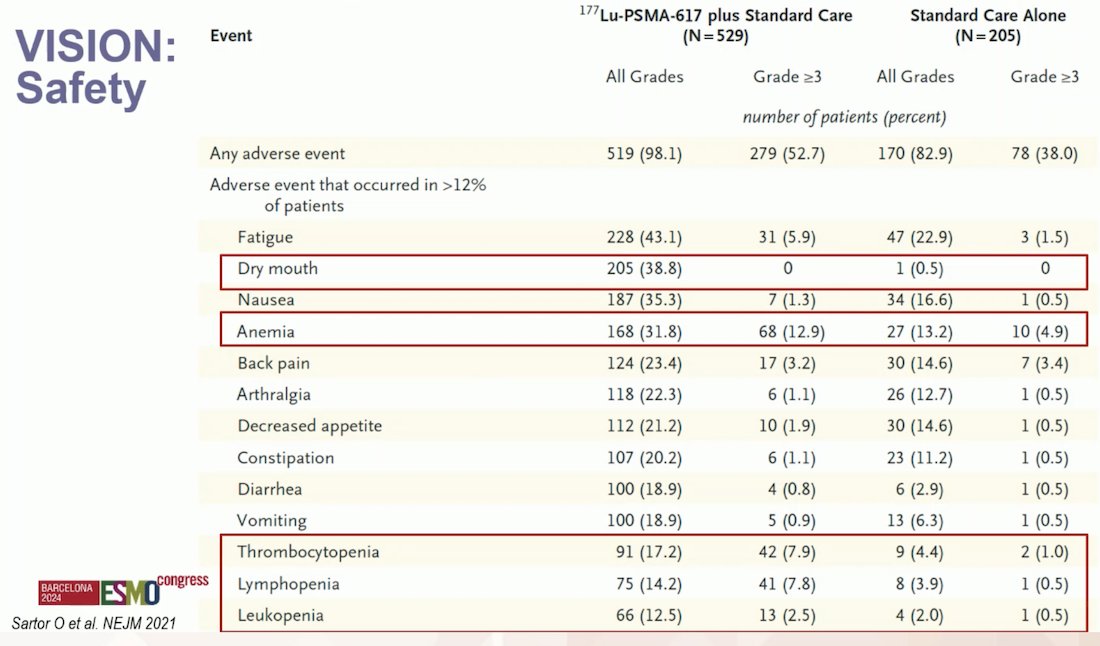
In terms of safety, in the VISION trial, the incidence of any-grade treatment-emergent adverse events (TEAEs) was consistent across the initial and extended cycles of 177Lu-PSMA-617, with no new safety concerns reported for patients receiving more than four cycles. Although TEAE incidence was higher with 177Lu-PSMA-617 compared to standard of care (SoC), the safety profile remained favorable. Longer exposure did not increase toxicity risk, supporting the use of up to six cycles of 177Lu-PSMA-617 for patients who benefit from and tolerate the therapy well.3
The most common treatment-emergent adverse events (TEAEs) reported in the VISION trial were dry mouth (38.8%), fatigue (43.1%), and anemia (31.8%, with Grade 3 anemia occurring in 12.9% of patients). Cytopenias were also prevalent, with thrombocytopenia affecting 17% of patients, lymphopenia 14%, and leukopenia 12.5%. Full details are shown in the table below.
Continuing in the third-line setting of mCRPC, Dr. Gillessen discussed the TheraP trial. This open-label, randomized phase 2 trial involved patients with mCRPC who had progressed after docetaxel and ARPI. Participants were selected based on PET imaging with 68Ga-PSMA-11 and 18F-FDG, showing PSMA-positive disease with no sites of metastatic disease exhibiting discordant 18F-FDG-positive and PSMA-negative findings. They were randomly assigned to receive either 177Lu-PSMA-617 every 6 weeks for up to six cycles or cabazitaxel (20 mg/m² every 3 weeks, up to ten cycles). The trial aimed to compare the efficacy and safety of these treatments.4

In the TheraP trial, inclusion criteria required 68Ga-PSMA PET positivity with a SUVmax of at least 20 at a disease site and >10 at all other lesions, with no discordant lesions on 18F-FDG PET. As a result, 10% of patients were excluded based on 68Ga-PSMA PET findings, and 17.5% were excluded due to discordant disease on 18F-FDG PET, totaling 27.5% of patients excluded based on both PET scans.
The TheraP trial found a positive primary endpoint for PSA response favoring 177Lu-PSMA-617. However, overall survival (OS) was comparable between 177Lu-PSMA-617 and cabazitaxel (19.1 months vs. 19.6 months, p = 0.77). Additionally, 177Lu-PSMA-617 was associated with fewer Grade 3/4 adverse events compared to cabazitaxel.4
Dr. Gillessen highlighted the key differences between the VISION and TheraP trials. In VISION, the control arm received standard of care, including treatments like ARPIs and taxanes, whereas in TheraP, the control arm was treated with cabazitaxel specifically. Notably, in TheraP, all participants had previously received docetaxel, and 15% had been treated with both abiraterone and enzalutamide. In contrast, VISION included 41% of patients who had received two types of taxanes and 49% who had been on two ARPIs. A detailed comparison between the trials is provided below. Both the VISION and TheraP trials demonstrated a similar overall response rate (ORR): 49% in TheraP and 51% in VISION.

Dr. Gillessen then discussed the PSMAfore trial, a phase 3 study evaluating 177Lu-PSMA-617 in taxane-naive patients with mCRPC. Eligible patients had mCRPC, were candidates for a change in ARPI after one progression on prior ARPI, and had ≥1 PSMA-positive lesion with no PSMA-negative lesions based on 68Ga-PSMA-11 PET/CT. Key exclusions included candidates for PARP inhibition, and those with prior systemic radiotherapy, immunotherapy, or chemotherapy within the last 6 months. Patients were randomized 1:1 to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or a change in ARPI (abiraterone or enzalutamide). Patients randomized to ARPI could crossover to 177Lu-PSMA-617 upon centrally reviewed radiographic progression. The trial design for PSMAfore was as follows:

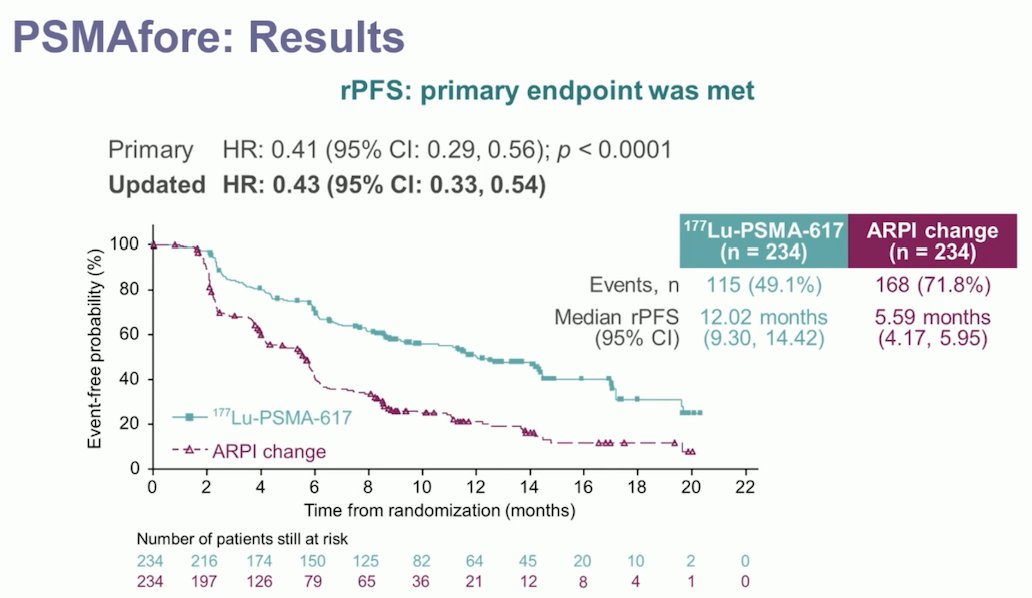
The PSMAfore trial met its primary endpoint, demonstrating a significant improvement in radiological progression-free survival (rPFS). The updated hazard ratio was 0.43 (95% CI 0.33-0.54) favoring 177Lu-PSMA-617, as illustrated in the Kaplan-Meier curve below.
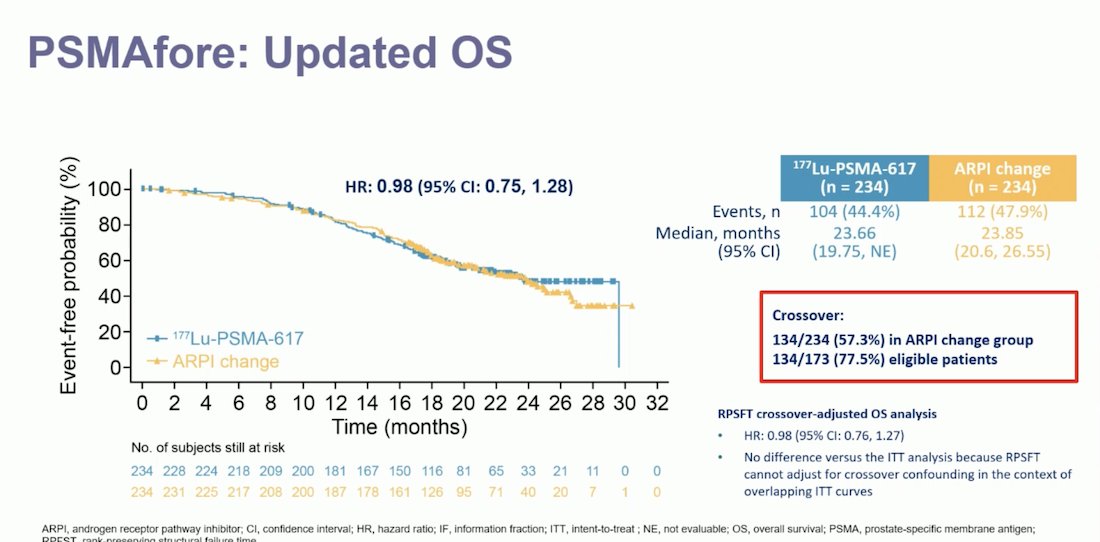
Notably, despite meeting its primary endpoint, the updated analysis of OS in the PSMAfore trial did not show a significant difference between patients treated with 177Lu-PSMA-617 and those who had an ARPI change (HR 0.98, 95% CI 0.75-1.28). Dr. Gillessen noted that this could be explained by the high crossover rate in the ARPI change group. A total of 134 out of 234 patients (57.3%) in the ARPI change group crossed over to radioligand therapy, and 77.5% of eligible patients participated in the crossover. Despite this, after adjusting for the crossover, the crossover-adjusted OS analysis showed a HR of 0.98 (95% CI 0.76-1.27).
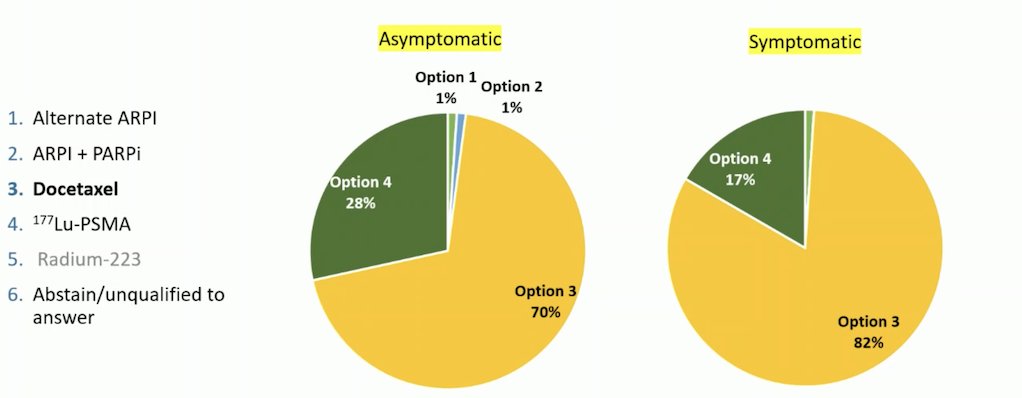
Dr. Gillessen presented data from the Advanced Prostate Cancer Consensus Conference (APCCC) in 2024. When asked about the preferred treatment strategy for chemotherapy-fit patients with PSMA-positive mCRPC who met the criteria for 177Lu-PSMA-617 therapy and had received a prior line of ARPI but no chemotherapy, the audience largely favored docetaxel. For symptomatic patients, 82% of the audience voted for docetaxel, while for asymptomatic patients, 70% preferred docetaxel. However, 28% of the voters chose 177Lu-PSMA-617 as a treatment option for asymptomatic patients, and 17% favored it for symptomatic patients.
She briefly mentioned the SPLASH trial, which evaluates the efficacy of 177Lu-PNT2002 in PSMA-positive mCRPC patients after a prior line of ARPI. The results of this trial are highly anticipated and the presentation is scheduled for Sunday.
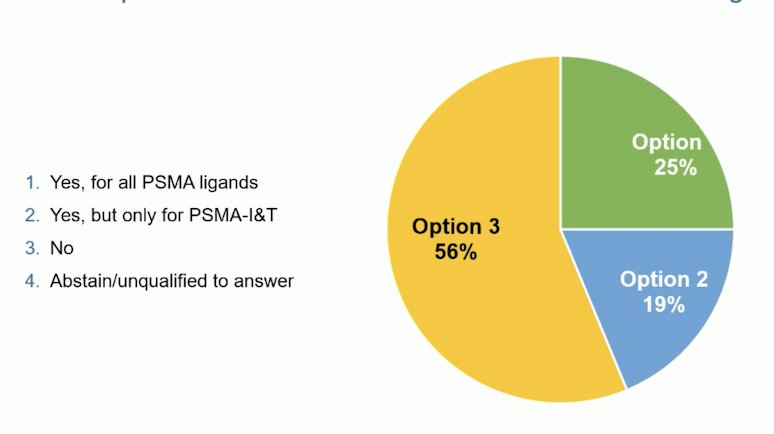
Furthermore, at the APCCC 2024, the audience was asked whether the data from PSMAfore and VISION could be extrapolated to Lutetium-PSMA with alternate PSMA ligands. The majority of the audience voted "No"; however, a quarter of the auditorium voted "Yes," but only for PSMA-I&T.
Dr. Gillessen moved on to discuss how to better select patients for treatment with 177Lu-PSMA. She presented a post-hoc analysis of the VISION trial that evaluated the associations of quantitative PET parameters with rPFS, OS, and ORR. The authors reported that whole-body tumor SUVmean was the best predictor of 177Lu-PSMA-617 efficacy, with a hazard ratio (HR) range of 0.86-1.43 for all outcomes (all p< 0.001).6
The UpFrontPSMA study, a randomized Phase 2 trial comparing sequential 177Lu-PSMA-617 plus docetaxel versus docetaxel alone in metastatic hormone-sensitive prostate cancer (mHSPC), will be presented by Arun Azad on Sunday at ESMO. This study aims to address whether earlier use of radioligand therapy is beneficial.
The dose and schedule of 177LuPSMA have varied between the different trials:
- VISION trial: 4 cycles and 2 additional cycles in patients with evidence of response (Dose: 7.4 GBq every 6 weeks)
- TheraP: Up to 6 cylces, treatment suspended when SPECT CT showed low or no PSMA uptake at the site of metastatic lesions (Dose: 8.5 GBq, decreased by 0•5 GBq per cycle)
- SPLASH trial: 4 cycles (Dose: 6.8 GBq every 8 weeks)
Dr. Gillesen presented data from the APCCC, showing that the majority of the audience (76%) would opt to complete all 6 cycles of 177Lu-PSMA if there was a good response (PSA, clinical, or radiological), even if PSMA-based imaging revealed significant remaining uptake. However if there was no remaining uptake 57% of the voters chose to stop at 4 cycles of 177LuPSMA therapy.

The question is how to monitor your patients who underwent treatment with 177LuPSMA. She discussed two strategies, one proposed by the VISION trial and the other one proposed by the TheraP trial:
- VISION: CT or MRI and bone scans every 8 weeks for 24 weeks and then every 12 weeks
- TheraP: CT of the chest, abdomen and pelvis, and bone scans every 12 weeks.
Dr. Gillesen concluded her presentation by stating that 177Lu-PSMA is now considered a standard of care for patients with metastatic castration-resistant prostate cancer (mCRPC) after docetaxel and an ARPI, based on the VISION trial. It is also an option for selected patients (those not candidates for PARPi, not fit for or unwilling to undergo chemotherapy but with ECOG PS 0-1) with mCRPC after an ARPI, based on the PSMAfore trial, provided they meet the study criteria.
We have an effective new treatment option, but there is still room for improvement. Future efforts should focus on optimizing the dose and schedule of 177Lu-PSMA, evaluating alpha-emitters, refining patient selection and biomarkers, and combining this therapy with other effective treatments.
Presented by: Silke Gillessen Sommer, MD, Medical Oncologist, Medical and Scientific Director, L'Istituto Oncologico della Svizzera Italiana (IOSI), Bellinzona, Switzerland
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Fizazi K, Gillessen S; ESMO Guidelines Committee. Electronic address: . Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann Oncol. 2023 Jun;34(6):557-563. doi: 10.1016/j.annonc.2023.02.015. Epub 2023 Mar 21. PMID: 36958590.
- Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ; VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103. doi: 10.1056/NEJMoa2107322. Epub 2021 Jun 23. PMID: 34161051; PMCID: PMC8446332.
- Chi KN, Armstrong AJ, Krause BJ, Herrmann K, Rahbar K, de Bono JS, Adra N, Garje R, Michalski JM, Kempel MM, Fizazi K, Morris MJ, Sartor O, Brackman M, DeSilvio M, Wilke C, Holder G, Tagawa ST. Safety Analyses of the Phase 3 VISION Trial of [177Lu]Lu-PSMA-617 in Patients with Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2024 Apr;85(4):382-391. doi: 10.1016/j.eururo.2023.12.004. Epub 2024 Jan 6. PMID: 38185538.
- Hofman MS, Emmett L, Sandhu S, Iravani A, Buteau JP, Joshua AM, Goh JC, Pattison DA, Tan TH, Kirkwood ID, Ng S, Francis RJ, Gedye C, Rutherford NK, Weickhardt A, Scott AM, Lee ST, Kwan EM, Azad AA, Ramdave S, Redfern AD, Macdonald W, Guminski A, Hsiao E, Chua W, Lin P, Zhang AY, Stockler MR, Williams SG, Martin AJ, Davis ID; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024 Jan;25(1):99-107. doi: 10.1016/S1470-2045(23)00529-6. Epub 2023 Nov 30. PMID: 38043558.
- LBA13 Phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore) Sartor, O. et al. Annals of Oncology, Volume 34, S1324 - S1325
- Kuo PH, Morris MJ, Hesterman J, Kendi AT, Rahbar K, Wei XX, Fang B, Adra N, Garje R, Michalski JM, Chi K, de Bono J, Fizazi K, Krause B, Sartor O, Tagawa ST, Ghebremariam S, Brackman M, Wong CC, Catafau AM, Benson T, Armstrong AJ, Herrmann K. Quantitative 68Ga-PSMA-11 PET and Clinical Outcomes in Metastatic Castration-resistant Prostate Cancer Following 177Lu-PSMA-617 (VISION Trial). Radiology. 2024 Aug;312(2):e233460. doi: 10.1148/radiol.233460. PMID: 39162634; PMCID: PMC11366674.


