Currently, there are multiple treatments available for ARPI-resistant disease, including docetaxel, radioligand therapy, PARP inhibitors, and immune checkpoint inhibitors. The latter two are used for molecularly selected patients, while 177Lu-PSMA is used for patients selected via molecular imaging.
Dr. Attard discussed the STAMPEDE trial, an open-label Phase 3 study involving patients with high-risk non-metastatic prostate cancer. This was defined as node-positive disease or, if node-negative, having at least two of the following characteristics: tumor stage T3 or T4, Gleason sum score of 8-10, and prostate-specific antigen (PSA) concentration ≥40 ng/mL. The trial also included patients relapsing with high-risk features, such as ≤12 months of total ADT with an interval of ≥12 months without treatment, PSA concentration ≥4 ng/mL with a doubling time of <6 months, PSA concentration ≥20 ng/mL, or nodal relapse.
Local radiotherapy was mandated for node-negative patients and encouraged for node-positive disease. Patients were randomly assigned (1:1) to either ADT alone (control group) or ADT with abiraterone acetate and prednisolone (combination-therapy group). In a second trial, the combination-therapy group also received enzalutamide (160 mg daily orally). ADT was administered for 3 years, while combination therapy was given for 2 years.1
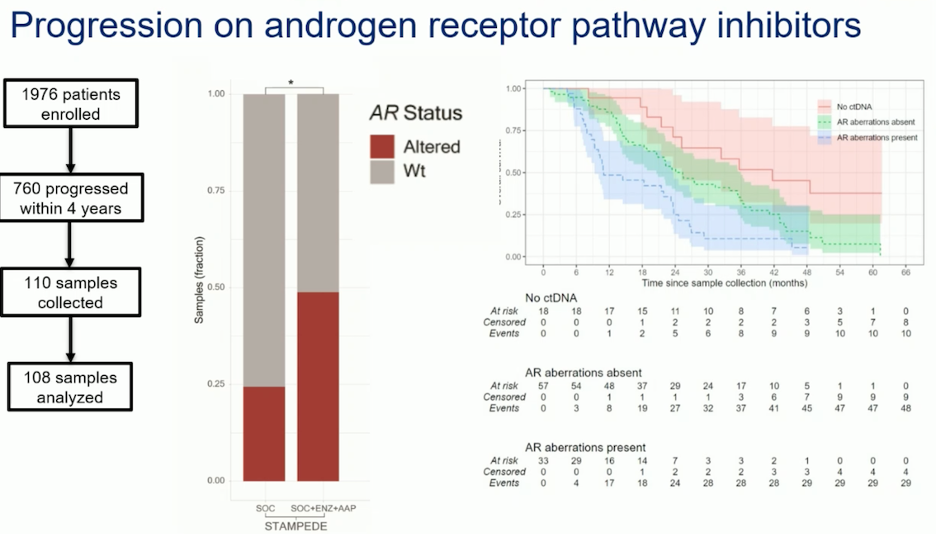
They collected and analyzed 108 samples from 760 patients in the STAMPEDE trial1 who had progressed while on ARPI. Their findings showed that among patients in the intensification group, up to 50% had mutations in the AR, while the remaining patients had wild-type AR status. In contrast, only 25% of patients in the ADT group had AR mutations, indicating that early initiation of ADT is not associated with AR mutations. The study also found that patients with undetectable ctDNA had better prognosis and survival. Additionally, patients without AR aberrations had superior survival compared to those with AR alterations, as shown in the graphic below. Therefore, AR alterations and ctDNA status are important prognostic indicators for survival in patients with prostate cancer.
Furthermore, they analyzed plasma samples from patients in the PRESIDE study. This was a multinational, double-blind, randomized, placebo-controlled Phase 3 trial. Patients were eligible if they had prostate adenocarcinoma without neuroendocrine differentiation or small-cell features, serum testosterone concentrations of 1.73 nmol/L or less, and had progressed during treatment with ADT. Patients received open-label enzalutamide 160 mg per day orally. At week 13, patients were assessed for either radiographic or PSA progression (defined as a 25% or more increase in PSA and a level 2 ng/mL or more above the nadir). Patients who showed a decline in PSA at week 13 but subsequently progressed were screened and enrolled in period 2 (P2). During P2, these patients were treated with up to ten cycles of intravenous docetaxel 75 mg/m² and were randomly assigned (1:1) to either oral enzalutamide 160 mg/day or oral placebo. The study found that up to 80% of patients who started enzalutamide for mCRPC had altered AR status, while the remaining had wild-type AR status. 2
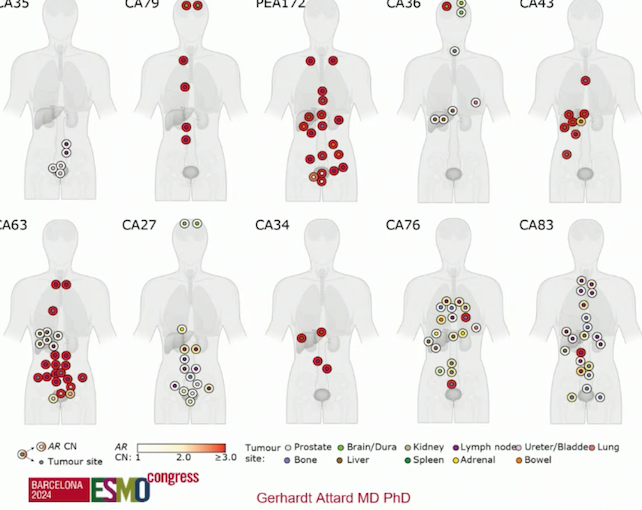
Dr. Attard and colleagues conducted genome-wide copy number profiling and bespoke analyses targeting the AR on 167 metastatic regions from 11 organs harvested post-mortem from 10 men who died from prostate cancer and had progressed on ARPI. In this study, every patient who progressed on ARPI with a rising PSA had AR gene alterations in more than one metastasis. Additionally, about 50% of patients also had concurrent clusters of AR wild-type metastases.3
As described by Dr. Castro in her talk, Dr. Attard emphasized that re-treatment with a second ARPI after progression on an ARPI should be avoided. He presented data from sequencing studies comparing abiraterone to enzalutamide, and enzalutamide to abiraterone, showing that regardless of the sequencing, PSA responses are not optimal (figure below). Therefore, he recommended against using ARPIs after progression on an initial ARPI.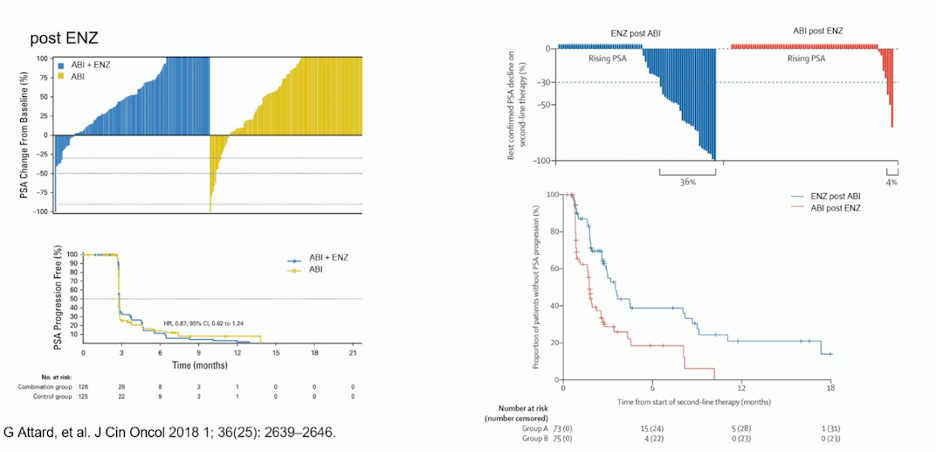
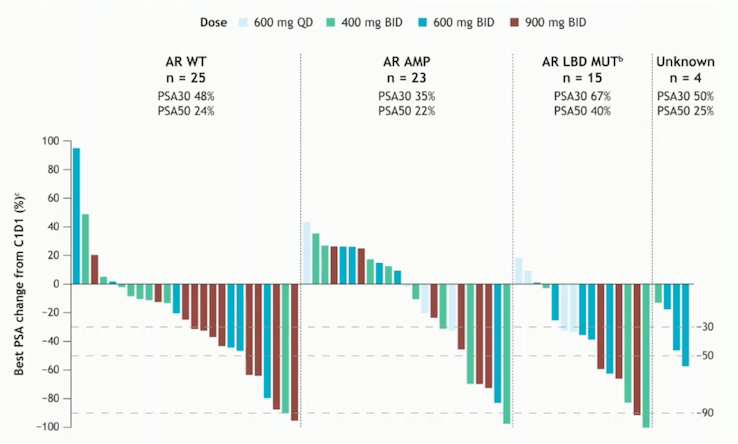
There are specific features associated with ARPI-resistant mCRPC, including persistent AR expression and active AR signaling in most cases, the selection of clones with AR gene alterations, and the limited effectiveness of sequential targeting of AR ligand-binding domain activation. Dr. Attard discussed third-generation ARPIs, such as ARV-766. This novel, potent, orally administered PROTAC (proteolysis-targeting chimeric) androgen receptor degrader targets wild-type AR as well as clinically relevant AR ligand-binding domain mutants, including the most prevalent AR L702H, H875Y, and T878A mutations. This novel ARPI increases degradation of the AR protein and can also act as an inhibitor that binds to the amino-terminal domain, potentially serving as an alternative approach to androgen synthesis inhibition.4
BSM-986365 (CC-94676) is another promising compound for ARPI-resistant mCRPC. This molecule recruits AR to the cereblon E3 ligase, facilitating target ubiquitination and proteasome-mediated protein degradation. It has demonstrated consistent PSA declines in cases with AR wild-type, AR amplifications, and AR ligand-binding domain mutations.5
PARP inhibition for ARPI-resistant mCRPC
Approximately 20% of mCRPC patients have alterations in genes involved in homologous recombination repair (HRR). The most common germline mutations in mCRPC are found in the BRCA2 gene (44%), followed by ATM (13%) and CHEK2 (12%). These alterations can result in the loss of gene function, loss of both copies, or other genetic changes. As shown in the slide below, when a BRCA alteration is present in the tumor (somatic), about 50% of patients will also have that alteration in the germline.
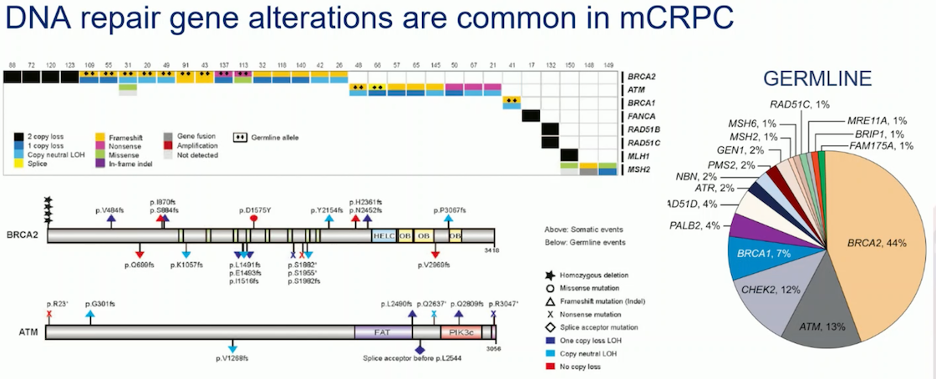
PARP inhibition in tumor cells with BRCA loss varies depending on the type of BRCA loss. Tumor cells with homozygous BRCA loss are generally more sensitive to PARP inhibitors compared to those with heterozygous BRCA mutations. There is a notable difference in sensitivity between these groups, with wild-type BRCA cells showing lower ensitivity to PARP inhibitors.
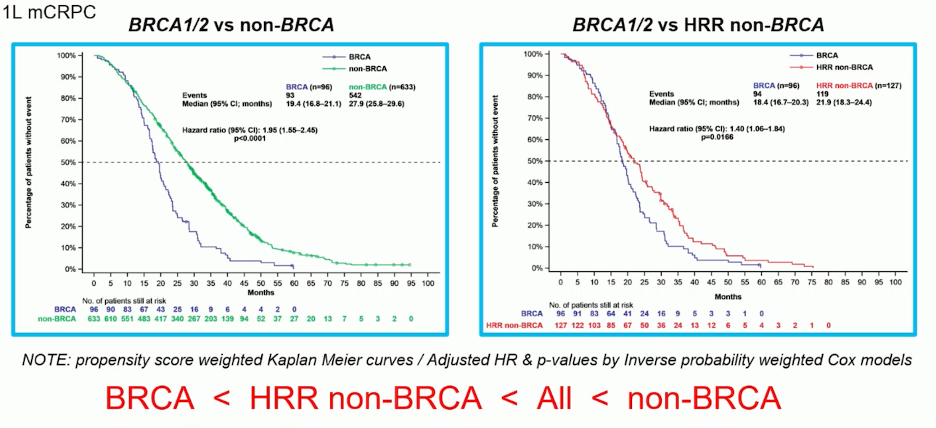
The clinical implications of BRCA mutations in the first-line setting of mCRPC have been examined in a study using data from 729 patients pooled from the CAPTURE study. The analysis revealed that patients with BRCA mutations had significantly worse radiographic progression-free survival (rPFS), progression-free survival 2 (PFS2), and overall survival (OS) compared to those with non-HRR mutations and non-BRCA patients. Propensity score-weighted Kaplan-Meier curves illustrating these outcomes are shown below.
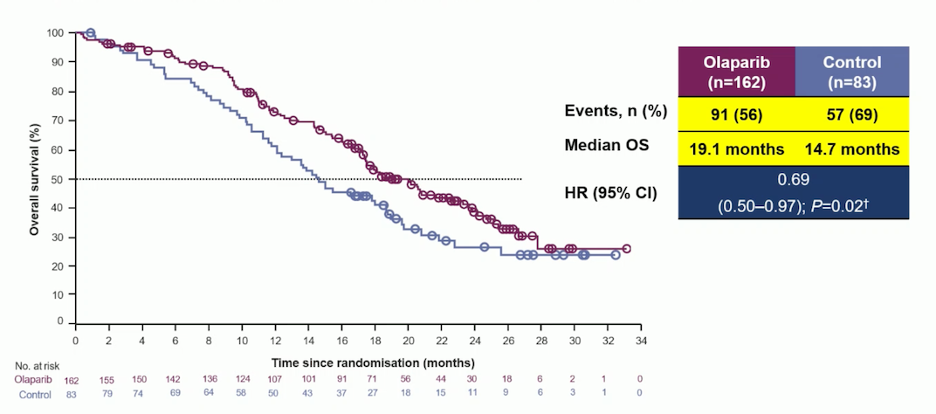
The PROfound study was a Phase III trial that enrolled men with mCRPC who had progressed on prior treatment with abiraterone acetate or enzalutamide, whether administered during non-metastatic castration-resistant prostate cancer or metastatic hormone-sensitive prostate cancer. Patients with previous taxane exposure were also included. The study focused on identifying alterations in one of 15 pre-specified genes involved in homologous recombination repair, including BRCA1/2, ATM, BRIP1, BARD1, CDK12, CHEK1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, and RAD54L. The trial compared the efficacy of the PARP inhibitor olaparib against ARPIs (enzalutamide or abiraterone). Results showed that the median overall survival (OS) was 19.1 months in the olaparib group, compared to 14.7 months in the ARPI group, with a hazard ratio for OS of 0.69 (p=0.02).7
TRITON 3 was a Phase III trial comparing rucaparib, a PARP inhibitor, with either a second ARPI or docetaxel in patients with ARPI-resistant mCRPC and BRCA1/2 loss. The trial demonstrated that rucaparib significantly improved progression-free survival compared to docetaxel (hazard ratio [HR] 0.53, 95% confidence interval [CI] 0.37–0.77) and to a change in ARPI (HR 0.38, 95% CI 0.25–0.58).8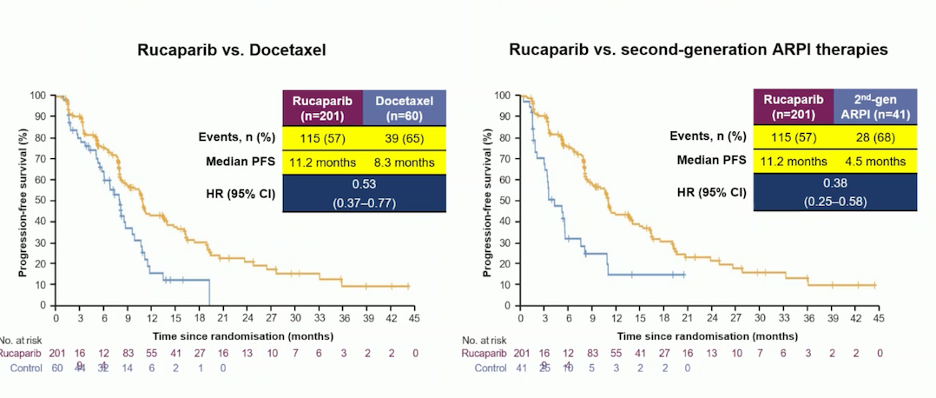
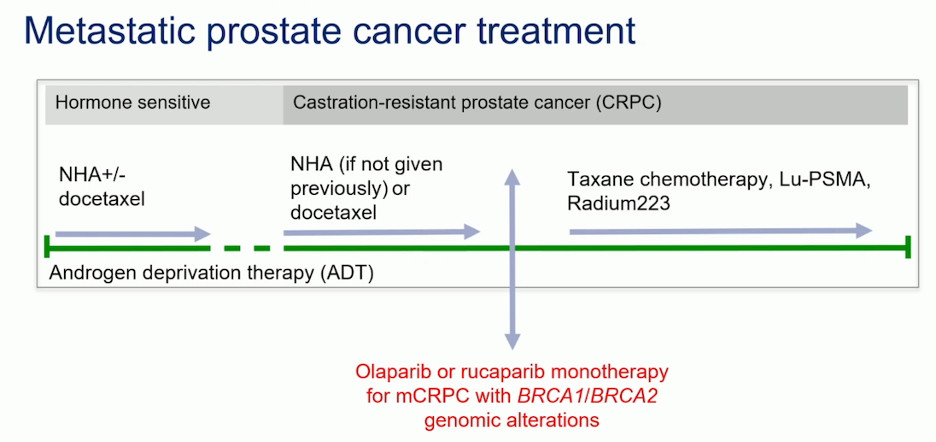
Dr. Attard presented a timeline for prescribing olaparib or rucaparib monotherapy in mCRPC patients with BRCA1/BRCA2 mutations, based on findings from the PROfound and TRITON 3 trials. He emphasized that this approach should be well established in clinical practice, recommending that patients with ARPI-resistant mCRPC and BRCA1/2 alterations be treated with PARP inhibitors.
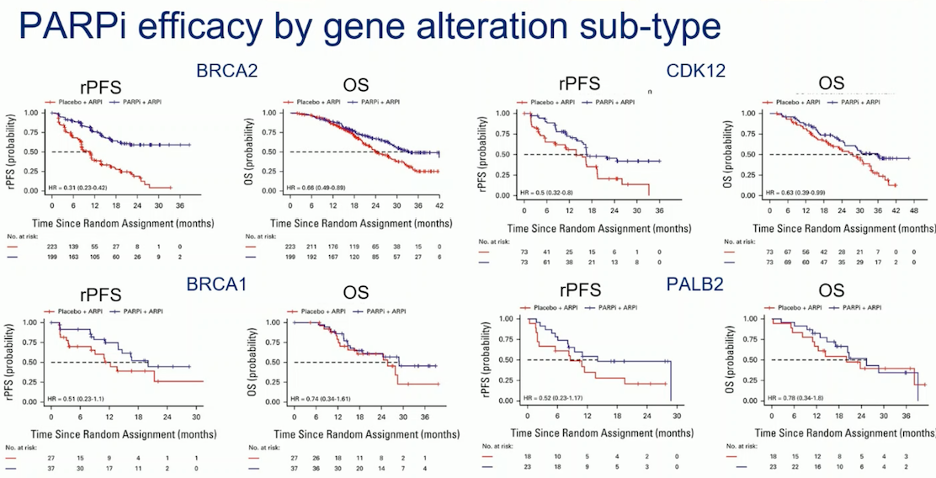
In a pooled analysis of patient-level data from trials of PARPi in mCRPC, which reported mutation status in individual HRR genes, BRCA2 patients treated with PARPi and ARPI in the first-line setting experienced significantly improved rPFS and OS. In contrast, patients with BRCA1 mutations did not show significant improvements in either rPFS or OS with the combination. The benefit from PARPi appeared greatest for patients with BRCA1m, BRCA2m, CDK12m, and PALB2m, though only BRCA2m demonstrated a significant improvement in OS. However, the subgroups are underpowered, and variability in test sensitivity across different trials—such as plasma versus tissue analyses—may impact the results.9
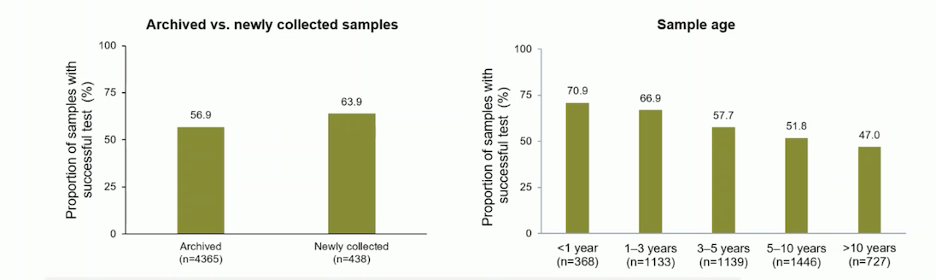
In the Phase III PROfound study, an investigational clinical trial assay based on the FoundationOneCDx tissue test was used to prospectively identify patients with qualifying HRR gene alterations. The study analyzed 4,858 tissue samples from 4,047 patients, with nearly 17% being metastatic tumor samples (60% archival and 33% newly obtained). NGS results were more frequently generated from newly collected samples compared to archival samples (63.9% vs. 56.9%), and from metastatic samples compared to primary samples (63.9% vs. 56.2%). The generation of NGS results declined with increasing sample age, with approximately 50% of samples older than 10 years providing results. Dr. Attard emphasized that archived samples were more likely to fail at the stage of DNA extraction, whereas newly collected samples were most likely to fail at pathology review. He highlighted the importance of analyzing samples at earlier stages of the disease.10
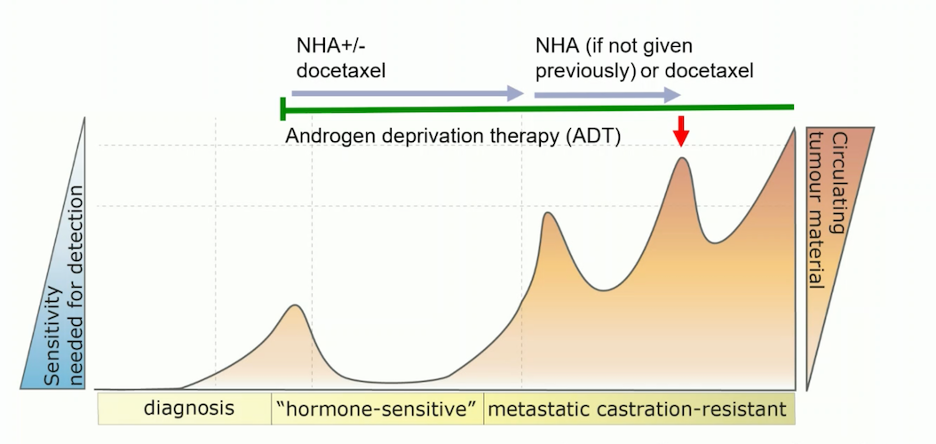
Additionally, Dr. Attard noted that ctDNA is most effective in the later stages of the disease, specifically in mCRPC after treatment with ARPI +/- docetaxel or ARPI alone, due to the higher amount of ctDNA tumor fraction.
Lastly, detection sensitivity of ctDNA% in a cell-free DNA (cfDNA) sample relies on the type of alteration. In patients with mutations, the sensitivity is >0.01%; for monoallelic deletions, it is >5-10%; for biallelic deletions, it is <10-15%; and for amplifications, it is 2-5%. A cfDNA assay can fail to detect a somatic alteration if the ctDNA fraction is very low. However, a well-designed panel allows differentiation between no tumor presence and a negative result.
Immune checkpoint inhibition for mCRPC
Mismatch repair (MMR) deficiency gene alterations are present in approximately 2% of patients with mCRPC. The most common MMR deficient genes are MSH2 and MLH1.11 Several trials in mCRPC are comparing immune checkpoint inhibitors (ICI) with different treatments, but with no benefit in unselected populations. Dr Attard believes ICIs do not have a role in unselected populations.
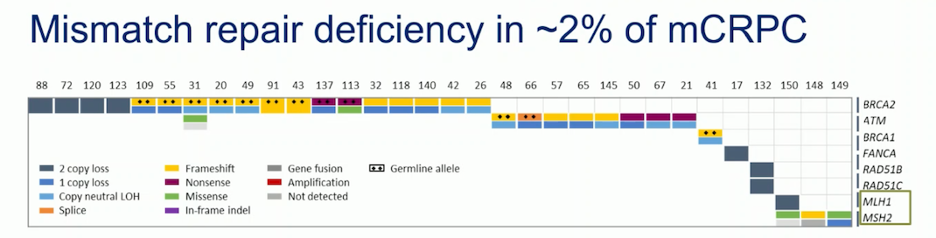
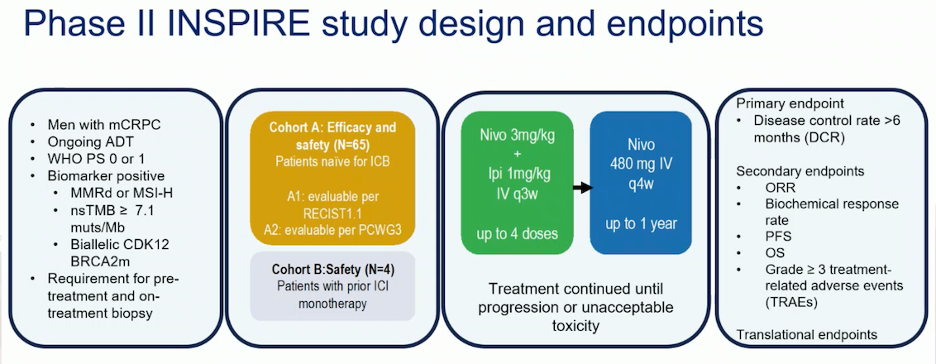
The INSPIRE study, a phase II trial in men with mCRPC who are biomarker positive (MMRd or microsatellite instability-high (MSI-H)), non-synonymous tumor mutational burden (nsTMB) ≥ 7.1 muts/Mb, or biallelic CDK12 or BRCA2 mutations, enrolled patients into two cohorts. Patients received nivolumab 3 mg/kg and ipilimumab 1 mg/kg (nivo3/ipi1), followed by nivolumab 480 mg IV every 4 weeks for up to 1 year. Dr. Mehra will present the findings of this study at ESMO.
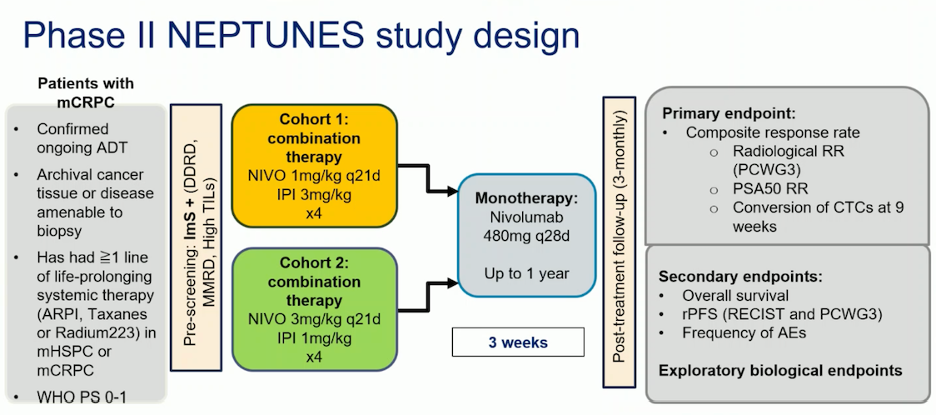
Another phase II trial, the NEPTUNES trial, included patients with mCRPC who had received ≥1 line of life-prolonging systemic therapy (ARPI, taxanes, or radium-223) in mHSPC or mCRPC. This trial examined two regimens: nivolumab 1 mg/kg and ipilimumab 3 mg/kg for four doses, versus nivolumab 3 mg/kg plus ipilimumab 1 mg/kg for four doses, followed by nivolumab monotherapy for up to 1 year. Patients were screened for multiple biomarkers, including MMRd, BRCA1/2, CDK12, ATM, and CHEK2. The study design is outlined below:
In the final analysis of NEPTUNES, patients with high MMRd demonstrated significantly higher response rates (80%) in mCRPC. Additionally, there were notable responses in patients with BRCA1/2 mutations (response rate 50%) and those with high inflammatory infiltrate (43%).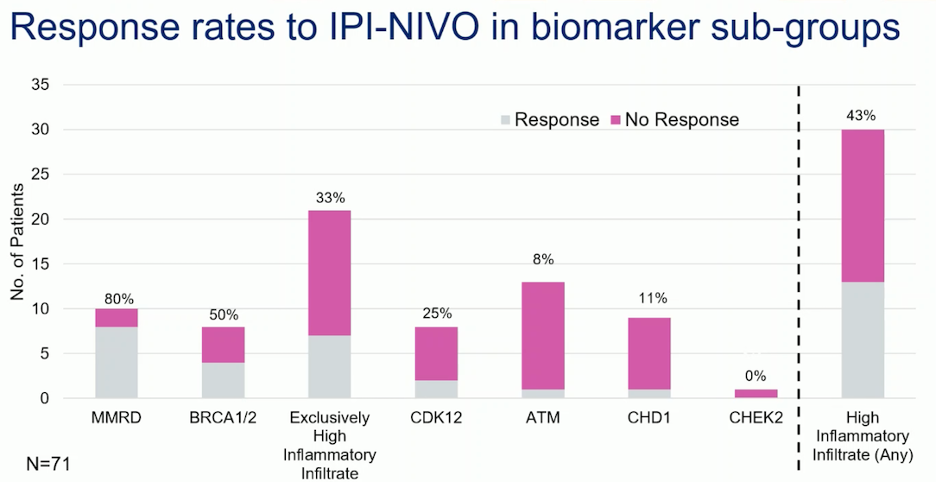
Dr Attard concluded his presentation with the following messages:
- All post-ARPI patients sufficiently fit for further treatment should undergo molecular testing
- Clear actionable targets for ARPI-resistant mCRPC are:
- BRCA2/1m →PARPi
- MMRd → immune checkpoint inhibition
- Additional molecular sub-groups that require further validation (CDK12m → PARPi)
- Tumor inflammatory infiltrates → immune checkpoint inhibition
Presented by: Gerhardt Attard MD, Ph.D., FRCP, John Black Charitable Foundation Endowed Chair in Urological Cancer Research, University College London Cancer Institute, London, UK
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto.
@chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, Gillessen S, Cook A, Brawley C, Amos CL, Atako N, Pugh C, Buckner M, Chowdhury S, Malik Z, Russell JM, Gilson C, Rush H, Bowen J, Lydon A, Pedley I, O'Sullivan JM, Birtle A, Gale J, Srihari N, Thomas C, Tanguay J, Wagstaff J, Das P, Gray E, Alzoueb M, Parikh O, Robinson A, Syndikus I, Wylie J, Zarkar A, Thalmann G, de Bono JS, Dearnaley DP, Mason MD, Gilbert D, Langley RE, Millman R, Matheson D, Sydes MR, Brown LC, Parmar MKB, James ND; Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022 Jan 29;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5. Epub 2021 Dec 23. PMID: 34953525; PMCID: PMC8811484.
- Merseburger AS, Attard G, Åström L, Matveev VB, Bracarda S, Esen A, Feyerabend S, Senkus E, López-Brea Piqueras M, Boysen G, Gourgioti G, Martins K, Chowdhury S. Continuous enzalutamide after progression of metastatic castration-resistant prostate cancer treated with docetaxel (PRESIDE): an international, randomised, phase 3b study. Lancet Oncol. 2022 Nov;23(11):1398-1408. doi: 10.1016/S1470-2045(22)00560-5. Epub 2022 Oct 18. PMID: 36265504.
- Hasan, A.M.M., Cremaschi, P., Wetterskog, D. et al. Copy number architectures define treatment-mediated selection of lethal prostate cancer clones. Nat Commun 14, 4823 (2023). https://doi.org/10.1038/s41467-023-40315-9
- Daniel P. Petrylak et al.ARV-766, a proteolysis targeting chimera (PROTAC) androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC): Initial results of a phase 1/2 study.. JCO 42, 5011-5011(2024). DOI:1200/JCO.2024.42.16_suppl.5011
- Dana E. Rathkopf et al.First-in-human phase 1 study of CC-94676, a first-in-class androgen receptor (AR) ligand-directed degrader (LDD), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC).. JCO 42, 134-134(2024) DOI:10.1200/JCO.2024.42.4_suppl.134
- Olmos D, Lorente D, Alameda D, Cattrini C, Romero-Laorden N, Lozano R, Lopez-Casas PP, Jambrina A, Capone C, Vanden Broecke AM, Trevisan M, Van Sanden S, Jürgens A, Herrera-Imbroda B, Castro E. Treatment patterns and outcomes in metastatic castration-resistant prostate cancer patients with and without somatic or germline alterations in homologous recombination repair genes. Ann Oncol. 2024 May;35(5):458-472. doi: 10.1016/j.annonc.2024.01.011. Epub 2024 Feb 27. PMID: 38417742.
- de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 May 28;382(22):2091-2102. doi: 10.1056/NEJMoa1911440. Epub 2020 Apr 28. PMID: 32343890.
- Fizazi K, Piulats JM, Reaume MN, Ostler P, McDermott R, Gingerich JR, Pintus E, Sridhar SS, Bambury RM, Emmenegger U, Lindberg H, Morris D, Nolè F, Staffurth J, Redfern C, Sáez MI, Abida W, Daugaard G, Heidenreich A, Krieger L, Sautois B, Loehr A, Despain D, Heyes CA, Watkins SP, Chowdhury S, Ryan CJ, Bryce AH; TRITON3 Investigators. Rucaparib or Physician's Choice in Metastatic Prostate Cancer. N Engl J Med. 2023 Feb 23;388(8):719-732. doi: 10.1056/NEJMoa2214676. Epub 2023 Feb 16. PMID: 36795891; PMCID: PMC10064172.
- Fallah J, Xu J, Weinstock C, Gao X, Heiss BL, Maguire WF, Chang E, Agrawal S, Tang S, Amiri-Kordestani L, Pazdur R, Kluetz PG, Suzman DL. Efficacy of Poly(ADP-ribose) Polymerase Inhibitors by Individual Genes in Homologous Recombination Repair Gene-Mutated Metastatic Castration-Resistant Prostate Cancer: A US Food and Drug Administration Pooled Analysis. J Clin Oncol. 2024 May 10;42(14):1687-1698. doi: 10.1200/JCO.23.02105. Epub 2024 Mar 14. PMID: 38484203; PMCID: PMC11095872.
- Hussain M, Corcoran C, Sibilla C, Fizazi K, Saad F, Shore N, Sandhu S, Mateo J, Olmos D, Mehra N, Kolinsky MP, Roubaud G, Özgüroǧlu M, Matsubara N, Gedye C, Choi YD, Padua C, Kohlmann A, Huisden R, Elvin JA, Kang J, Adelman CA, Allen A, Poehlein C, de Bono J. Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clin Cancer Res. 2022 Apr 14;28(8):1518-1530. doi: 10.1158/1078-0432.CCR-21-3940. PMID: 35091440.
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015 May 21;161(5):1215-1228. doi: 10.1016/j.cell.2015.05.001. Erratum in: Cell. 2015 Jul 16;162(2):454. PMID: 26000489; PMCID: PMC4484602.


